Anti-emetics in haemato-oncology patients with chemotherapy induced nausea and vomiting (CINV)
exp date isn't null, but text field is
Objectives
This guideline has been written to standardise the management of chemotherapy induced nausea and vomiting in children. It discusses the other causes of nausea and vomiting in this group of children, and also details the management of acute dystonic reactions.
Scope
This guideline should be in used in paediatric haemato-oncology patients who are receiving chemotherapy.
Nausea and vomiting are common and can be debilitating in children with cancer. The prevention of symptoms should be the goal of modern anti-emetic therapy. The risk of emesis depends on the emetogenicity of drugs (see Table 1- Appendix 1) and on the response of the patient. Oral and intravenous routes are equally efficacious providing that there are no barriers to drug absorption.
Selection of anti-emetic agents should be based on a sound, receptor based understanding of the pathophysiology of emesis. Common reasons for the failure of anti-emetic therapy in children are the selection of the wrong compound or the use of inadequate dosing.
The prescriber is responsible for calculating the dose of each anti-emetic drug for each admission/block of chemotherapy. It should not be assumed that the previously charted dose is correct.
CCLG guideline on the management of chemotherapy induced nausea and vomiting (Guidelines held in the Members area of the CCLG website - login required).
The management of patients with nausea and vomiting will be directed by the Consultant/Associate Specialist or a senior member of the medical team.
The Medical/Nursing team will be responsible for administration and monitoring.
Fluid balance charts and drug charts.
It should be remembered that patients receiving chemotherapy may have nausea or vomiting due to a cause other than the direct effect of the chemotherapy (CINV). These include:
- Radiotherapy (NB: TBI, gut or cranio-spinal are most likely to provoke emesis)
- Partial or complete bowel obstruction
- Vestibular dysfunction
- Brain metastases
- Electrolyte imbalance: hypercalcemia, hyperglycemia, hyponatremia
- Uraemia
- Concomitant drug treatments including opiates
- Gastroparesis
- tumour or chemotherapy (vincristine etc) induced
- Gastric Haemorrhage (coffee ground vomitus : may be steroid induced)
- Psychophysiologic:
- anxiety
- anticipatory nausea and vomiting
- Raised ICP
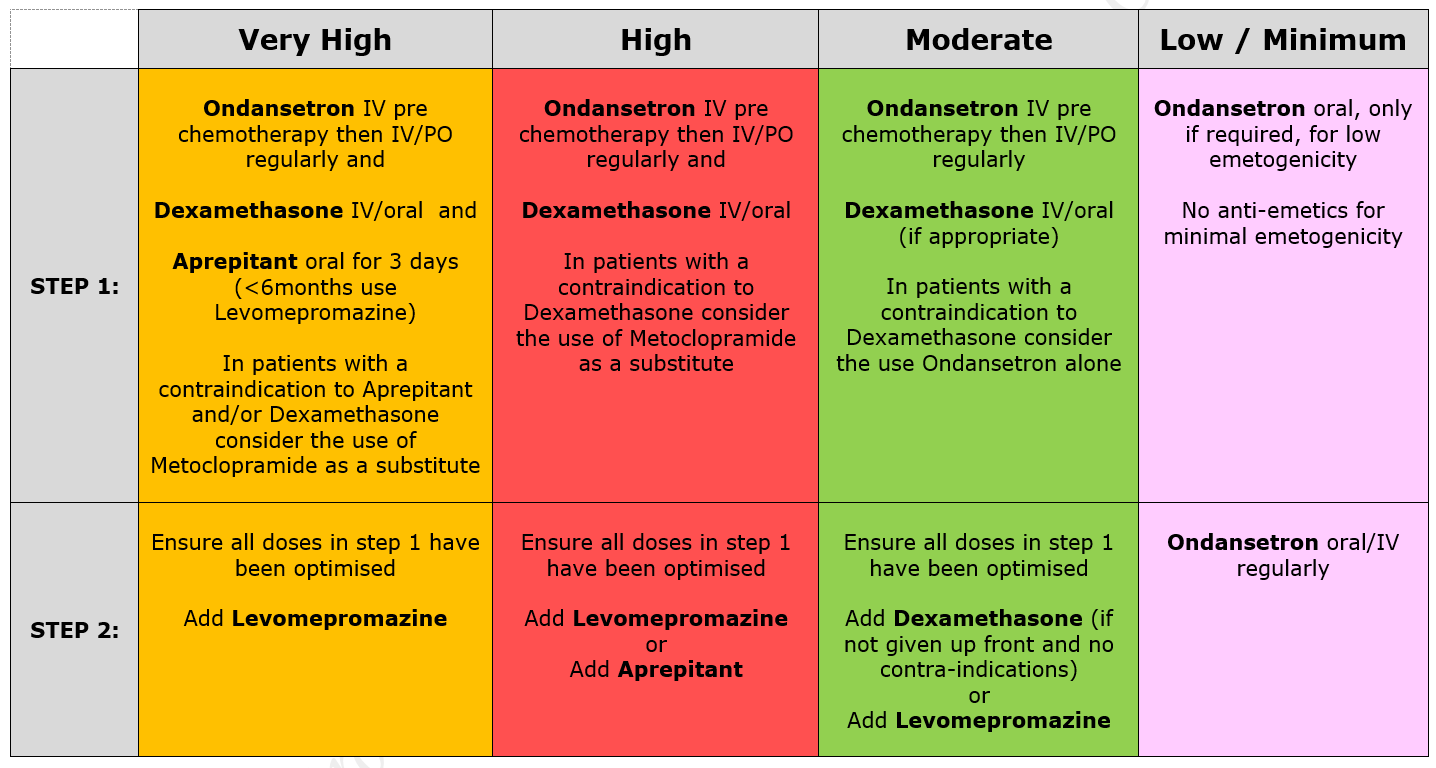
5.2.1 Patients receiving chemotherapy with a very high level of emetic risk should receive combination anti-emetic therapy with a neurokinin receptor antagonists (Aprepitant), a 5HT3 blocker (Ondansetron) and a corticosteroid (Dexamethasone). For chemotherapy with a high emetogenic potential, Ondansetron and Dexamethasone should be used as first line anti-emetic therapy, however, Aprepitant can be added to this combination as second-line therapy. Patients receiving moderately emetogenic chemotherapy will also require dual therapy with Ondansetron and Dexamethasone. Children receiving chemotherapy with a low emetic risk should receive only Ondansetron as required. If the chemotherapy agents have minimal emetogenicity, no routine prophylaxis is required. The choice of anti-emetic treatment detailed above assumes no contraindications to any of the drugs. Intravenous anti-emetics should be given at least 30 minutes prior to starting chemotherapy or orally at least 1 hr prior to starting treatment. Anti-emetics must be prescribed regularly on the appropriate patient’s prescription.
5.2.2 Dexamethasone should not be given to patients who receive steroids as part of their treatment protocol. Patients with Acute Lymphoblastic Leukaemia (ALL) receive Dexamethasone as part of induction and intensification therapy. Chemotherapy for ALL is not generally highly emetogenic and additional Dexamethasone for emesis should not be prescribed.
Patients with tumours within the Central Nervous System should not be started on Dexamethasone for as anti-emetic therapy as it may prevent chemotherapy penetrating the blood brain barrier.
Patients receiving immunomodulatory therapy should not receive steroids during that part of their treatment eg Neuroblastoma receiving Dinutuximab and Osteosarcoma receiving mifamurtide.
5.2.3 Dexamethasone should also be avoided in patients receiving conditioning treatment with chemotherapy and/or radiotherapy prior to stem cell transplantation, because steroid therapy will increase the risk of fungal infection in a population already at risk.
5.2.4 All children who face prolonged, profound neutropenia are at risk of invasive fungal infections and Dexamethasone should be avoided in these patients. This particularly relates to children with Acute Myeloid Leukaemia (AML) who are receiving, or have received, chemotherapy regimens containing Fludarabine and/or Gemtuzumab Ozogamicin (Mylotarg). After ensuring that Ondansetron has been prescribed at optimal dosing, failure of emetic control in these patients should be discussed with the consultant.
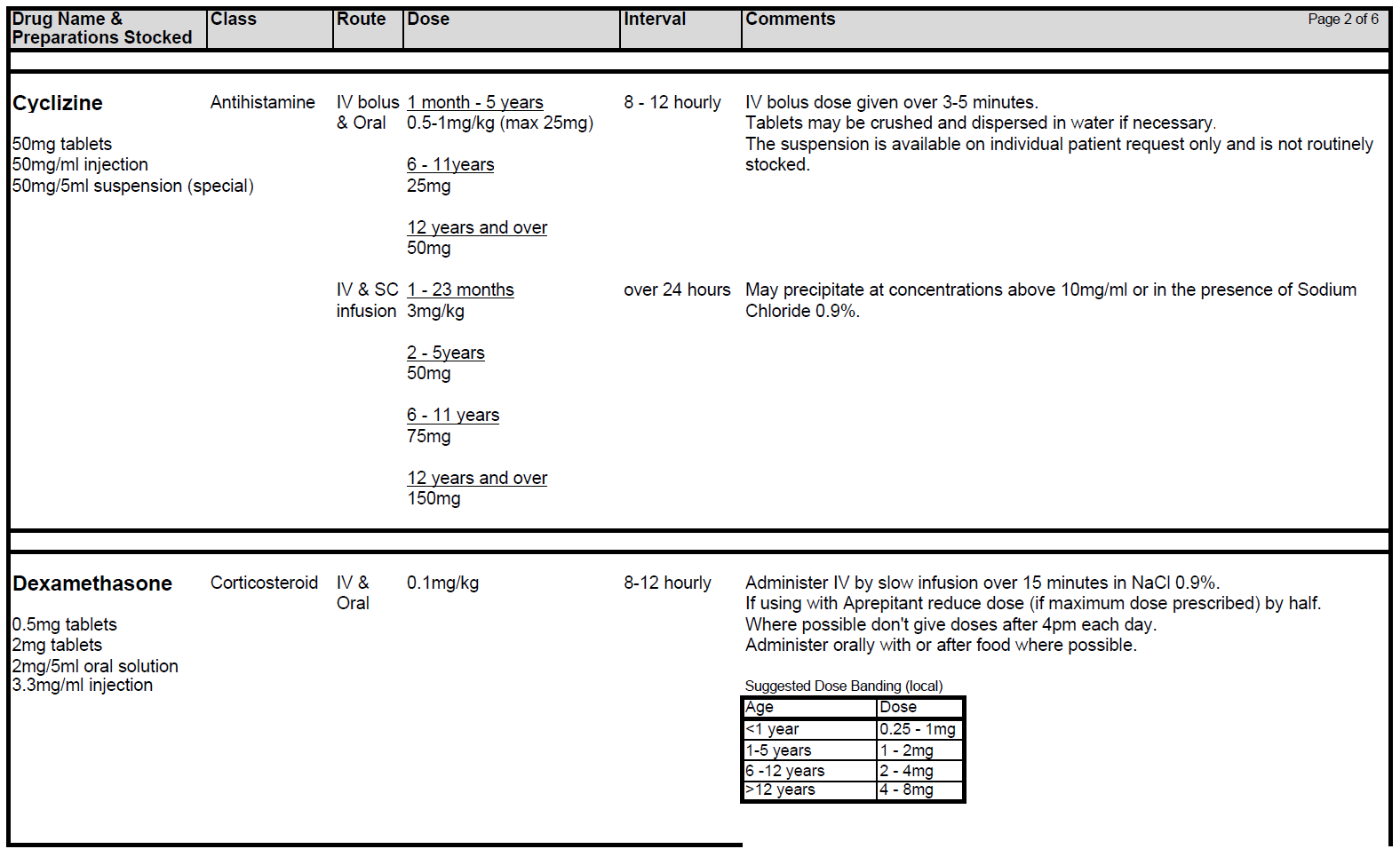
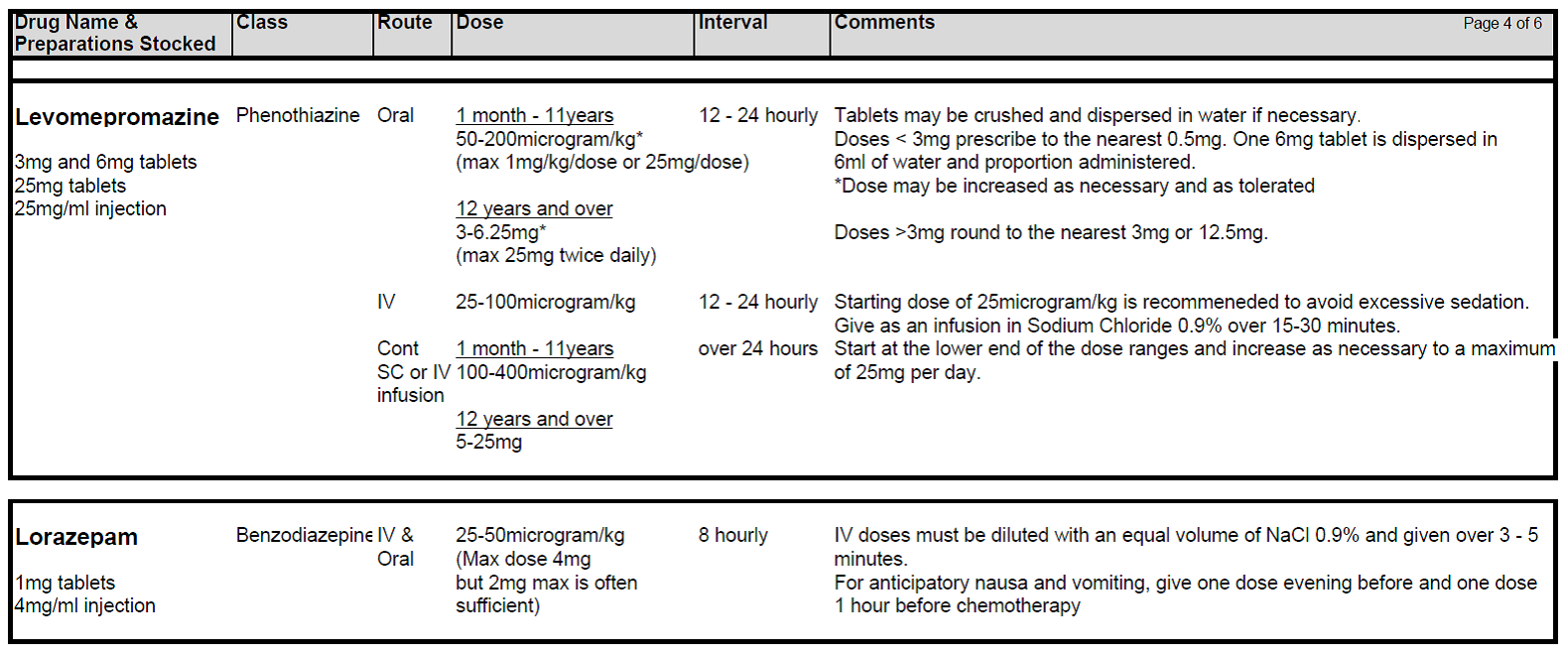
5.2.5 If standard anti-emetic therapy fails in any patient, exclude all other causes of nausea and/or vomiting and ensure anti-emetic drugs have been prescribed at optimal route, dosing and frequency. Check patient compliance and tolerance and consider if drug absorption might play a role in lack of response. If second line agents are considered necessary Levomepromazine is generally the drug of choice although both Metoclopramide and Cyclizine are also useful.
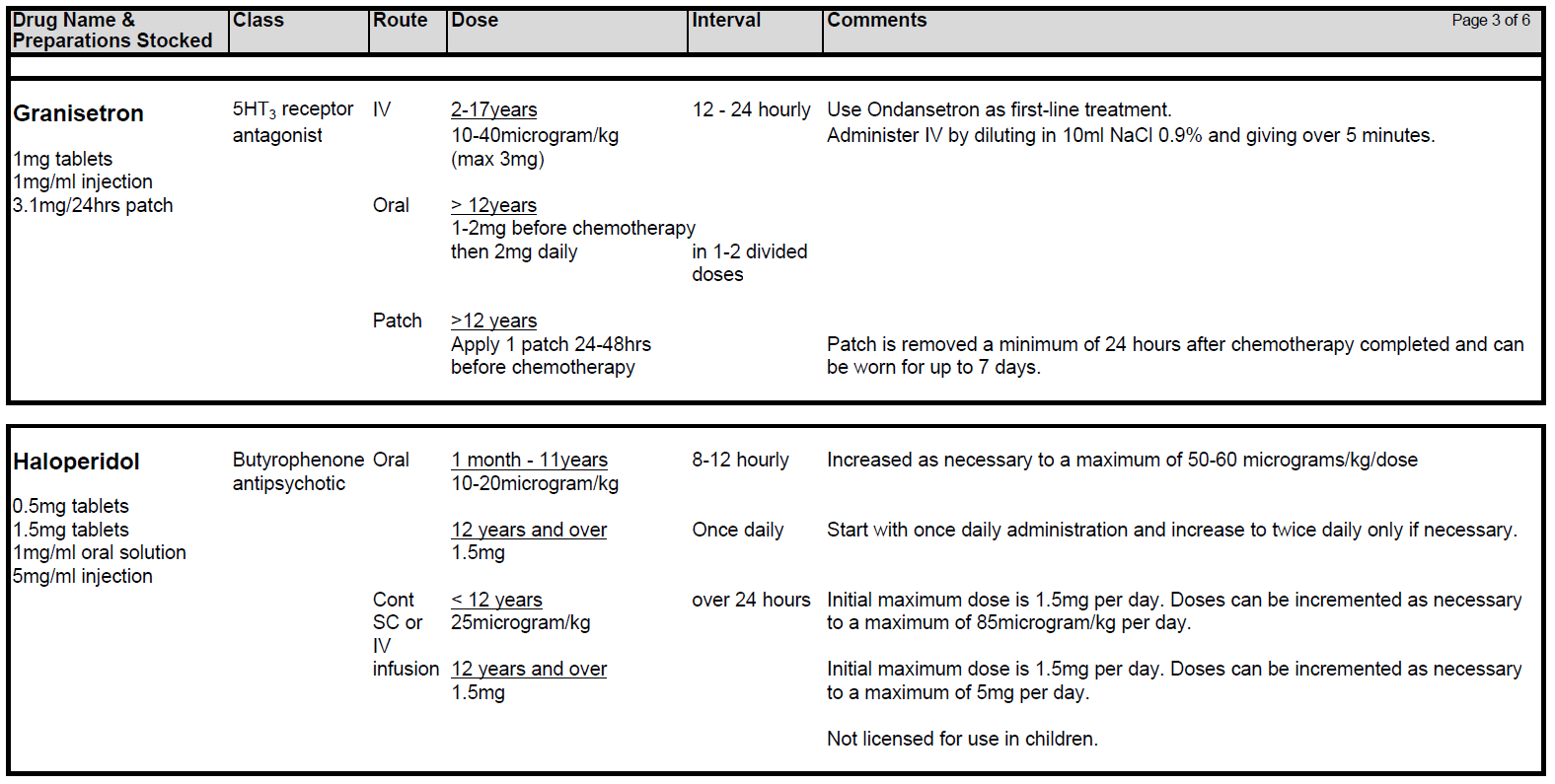
5.2.6 Levomepromazine (Nozinan) is a receptor non-specific agent that is often helpful in refractory cases although sedation can be problem. Beware of increased risk of extra-pyramidal side-effects and increased sedative effects when combining Metoclopramide with Cyclizine. Nabilone and Haloperidol may also be useful in certain circumstances.
5.2.7 Metoclopramide can be used in combination with Ondansetron for those receiving very highly or high emetogenic chemotherapy with a contraindication to Dexamethasone and/or Aprepitant. Metoclopramide is contra-indicated in children < 1 year. In children aged 1-18, Metoclopramide should be used as a second-line option for prevention of delayed chemotherapy-induced nausea and vomiting. Metoclopramide should only be prescribed for short-term use (<5 days). Avoid Cyclizine and Metoclopramide together – Metoclopramide is a prokinetic (stimulates the gut) while Cyclizine slows it down. They can be used together in a palliative care setting.
5.2.8 Consider using Lorazepam prior to a treatment block if either anticipatory nausea is a problem or anxiety forms a large component of the nauseating trigger (particular issue in adolescents). This may need to commence before starting the journey to hospital.
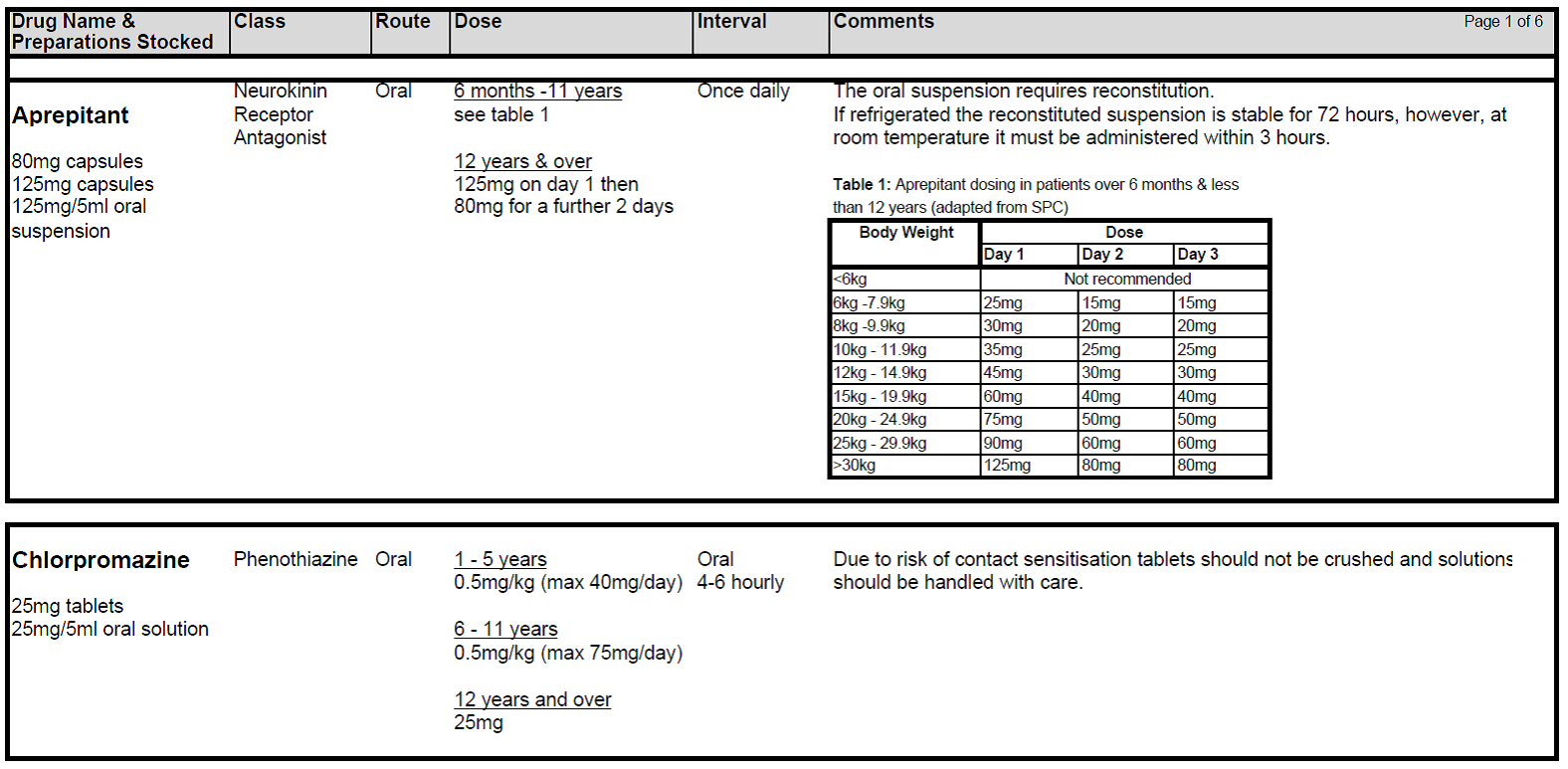
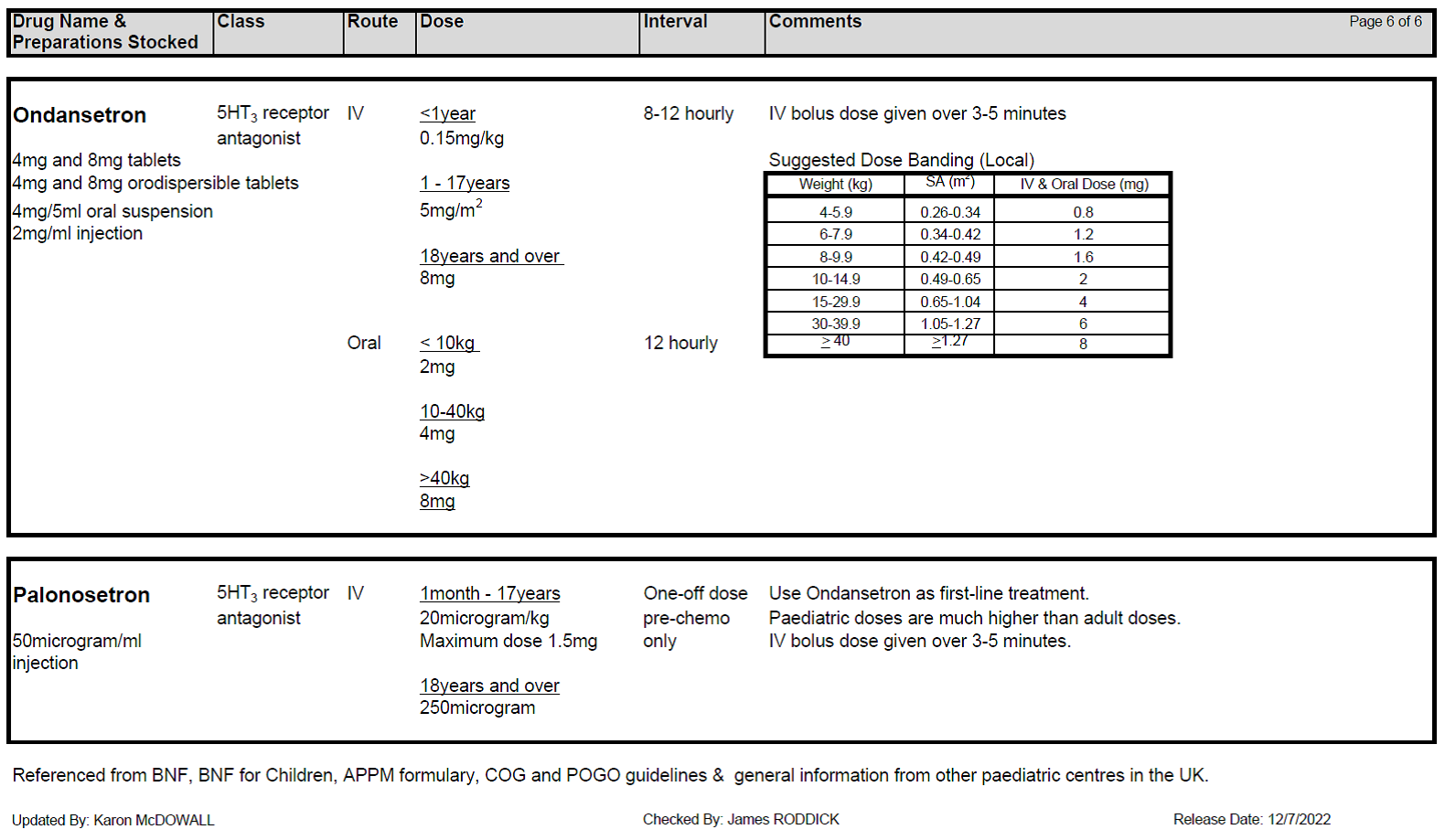
5.2.9 There is growing evidence that newer generation 5HT3 blockers eg Granisetron and Palonosetron may be of benefit in CINV. However these drugs should be discussed with a consultant and pharmacy prior to prescribing. Granisetron has been used successfully in a small number of patients in whom optimised Ondansetron treatment has failed. Aprepitant can be added to the anti-emetic regimen for high risk patients at the agreement of the Consultant.
5.2.10 Remember, anti-emetics have their own side effect profile (e.g. 5HT3 blockers are constipating and can cause headache).
NB: All episodes of refractory nausea and vomiting should be discussed with a consultant.
5.3.1 Acute Dystonic reactions may occur with Phenothiazines and Metoclopromide, especially if these drugs are used at high dose or in combination.
5.3.2 Treatment of dystonic reactions – Procyclidine 10mg in 2ml Injection (stocked on RHC 2A and 2B):
Dose:
Single IV bolus dose is usually effective within 5 – 10 minutes (up to 30 minutes). Can be repeated after 20 minutes.
| Age Group | Single IV bolus dose |
| 1 month - 1 year | 500 micrograms - 2 mg |
| 2-9 years | 2 - 5mg |
| ≥10 years | 5-10 mg |
When patients are discharged home following completion of a course of chemotherapy they should NOT be dispensed corticosteroid as an anti-emetic. Those requiring anti-emetics at home should be prescribed a maximum of TWO days of regular anti-emetic in the first instance and then reviewed. Pharmacy dispenses the patient’s original packs of anti-emetic drugs so it is essential that parents/carers/patients are counselled appropriately to take anti-emetics regularly for the first two days only following chemotherapy and then only if required thereafter.
Please refer to the CCLG Guideline on the management of CINV* for emetogenicity tables. The prescription for each course of treatment will have the emetogenicity defined within the Supportive Care section
Summary of Recommendations:
- Individual antineoplastic agents may have high, moderate, low or minimal emetogenic potential.
- The emetogenicity of multiple agent antineoplastic therapy given to children is classified based on the emetogenic potential of the most highly emetogenic agent in the combination to be given.
- The emetogenicity of multiple day antineoplastc therapy is classified in children based on the emetogenic potential of the most highly emetogenic agent on each day of therapy.
*Guidelines held in the Members area of the CCLG website - login required.

Last reviewed: 01 June 2023
Next review: 01 June 2025
Author(s): Dr D Murphy
Version: 4
Approved By: Sch Clin Gov Group & Hosp Governance Group
Document Id: RHC-HAEM-ONC-017