Anti-fungal policy (haematology/oncology)
exp date isn't null, but text field is
Objectives
This guideline has been written to assist specialists in the prophylaxis and management of invasive fungal infection in children being treated for haematological malignancies.
Scope
This guideline should be used by specialists caring for children with haematological malignancies and at risk of invasive fungal infections.
Invasive fungal infections (IFI) are an important cause of morbidity and mortality in patients with haematological malignancies, in particular those with prolonged and severe neutropenia. Treatment of invasive fungal infection with antifungal medicines is complicated in haemato-oncology patients due to the need for other potentially nephrotoxic or hepatotoxic medicines e.g. aminoglycosides, ciclosporin, tacrolimus and concomitant or potential nephrotoxic/hepatotoxic chemotherapy regimens.
2.1 IV/Oral drug kardexes
2.2 Drugs used in Haemopoietic Stem Cell Transplantation (CLIN-006)
3.1 The diagnosis and management of fungal disease will be directed by the HSCT Clinical Team.
3.2 The Medical/Nursing team will be responsible for monitoring & investigation.
4.1 Blood culture bottles
4.2 Bacteriology swabs
4.3 Universal container
4.4 Stool sample container
5.1 High Risk Group:
Patients with the following risk factors are at high risk of developing IFI:
- Acute leukaemia
- Neutrophil count of <0.5 x 10^9 /L for more than 2 weeks
- Patients receiving high steroids
- Recipients of allogeneic haematopoietic stem cell transplants
- GvHD
- Treatment with Fludarabine
- Treatment with Campath or ATG
- Previous fungal infection
- Fluconazole resistant colonisation
- Colonisation of more than one site plus neutropenia (neutrophils <1.0 x 109/L)
- Autograft where conditioning includes Total body irradiation
5.2 Diagnosis:
It is important that patients are regularly assessed for clinical features of IFI i.e:
- Daily physical examination
- Early radiological imaging following persistent pyrexia for more than 72-96 hours i.e. preferably before, but definitely within 48-72 hours of commencing antifungal therapy.
- In the first instance ultrasound of the liver and spleen. Consider early CT scanning of chest/sinuses if high clinical suspicion.
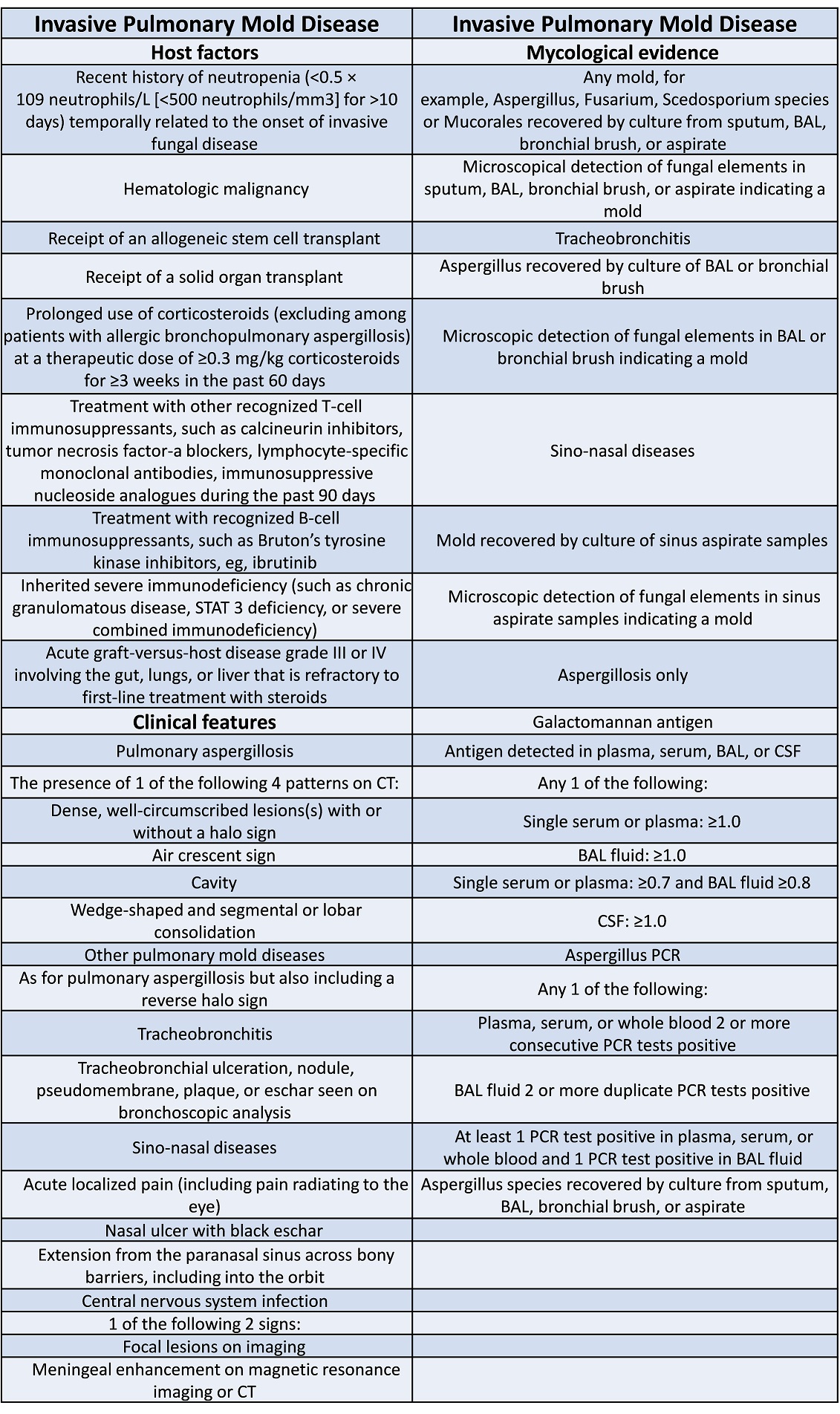
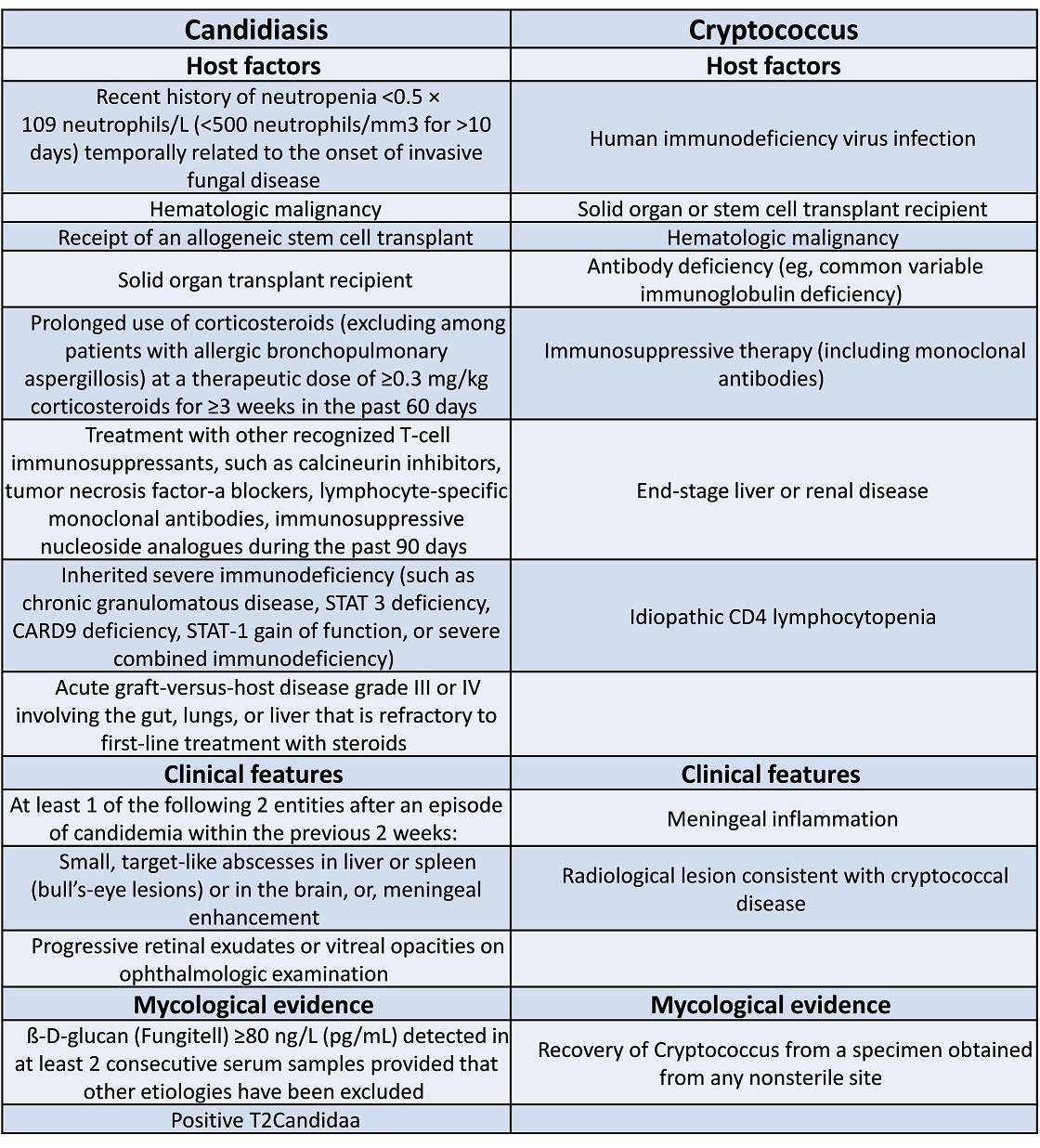
EORTC (European Organization for Research and Treatment of Cancer) criteria are helpful in determining the likelihood of proven/probable IFI
5.3 Monitoring of Renal Function:
Many antifungal medicines are nephrotoxic or hepatotoxic. Monitor serum creatinine daily and LFTs regularly during treatment.
5.4 Prophylaxis:
All haemopoietic stem cell transplant (HSCT) patients are commenced on IV Ambisome on the day of admission. It is prescribed on Mondays, Wednesdays and Fridays (dose: 2mg/kg/day). Selected oncology and haemato-oncology patients should also receive antifungal prophylaxis. In particular, children with relapsed acute lymphoblastic leukaemia (ALL), children undergoing induction therapy for acute lymphoblastic leukaemia and children receiving chemotherapy for acute myeloid leukaemia (AML) or lymphoma. Children with solid organ cancers should receive antifungal prophylaxis as recommended by individual protocols.
When engraftment is established and there are no signs of IFI Ambisome will be substituted with an oral azole, usually posaconazole. Anti-fungal prophylaxis should be discussed with senior members of the HSCT clinical team before being started. For doses see CLIN-006 SOP.
If not tolerating oral medicines or high risk for IFI:
- Ambisome 1mg/kg/day or 2mg/kg/day on Mondays, Wednesdays and Fridays.
If documented allergy to Ambisome:
- Caspofungin 50mg/m2 on alternate days
5.5 Empirical/Possible IFI Therapy:
Patients eligible for empirical therapy should either:
- Be in a high risk group with a pyrexia unresponsive to broad spectrum antibiotics for more than 96 hours
or
- fulfill the EORTC Criteria for Possible IFI - 1 host factor and 1 clinical factor as per EORTC risk factor table (Appendix 1) for invasive fungal infection.
Ambisome 3mg/kg/day (doses can be increased to 10mg/kg/day) in proven infections.
If allergic to Ambisome or impaired renal function:
Caspofungin: 70mg/m2 on D1, then 50mg/m2 once daily
5.6 Proven/Probable IFI Therapy:
- fulfill the EORTC Criteria for Probable IFI - 1 host factor, 1 clinical factor and 1 mycological factor as per EORTC risk factor table (Appendix 1) for invasive fungal infection.
Treatment drugs and doses as for empirical therapy (see section 5.5)
N.B: For individuals with evidence of intracerebral infection intravenous voriconazole is the drug of choice due to excellent penetration of the blood brain barrier
Voriconazole (by intravenous infusion):
- Child 2-12 yrs (and child 12-15 years if body weight under 50 kg): 9mg/kg every 12 hours for 2 doses then 8mg/kg every 12 hours (reduced in steps of 1mg/kg if not tolerated; increased in steps of 1mg/kg if inadequate response) for maximum period of 6 months.
- Child 15- 18 years (body weight over 50kg): 6mg/kg every 12 hours for 2 doses then 4mg/kg every 12 hours (reduced to 3mg/kg if not tolerated) for maximum period of 6 months.
5.7 Alternative Agents:
Posaconazole can be used to treat invasive aspergillosis which is unresponsive to Ambisome, or in patients intolerant to Ambisome, voriconazole or fluconazole (see YMBT-CLIN-0006 SOP for dosing and side-effects).
5.8 Additional Agents:
The addition of the following agents to antifungal therapy can be considered depending on the patients’ clinical condition.
G-CSF - Lenograstim 5mcg/kg equivalent dose, can be doubled to 10mcg/kg equivalent dose if required.
This SOP will be reviewed every two years.
For further information contact:
Haemopoietic Stem Cell Transplant Team on-call Consultant (via switchboard)
- Prentice A, Glasmacher A, et al. Guidelines on the management of invasive fungal infection during therapy for haematological malignancy (2007), BCSH
- Ben De Pauw, et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical Infectious Diseases, Volume 46, Issue 12, 15 June 2008, Pages 1813–1821
- J Peter Donnelly, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clinical Infectious Diseases, Volume 71, Issue 6, 15 September 2020, Pages 1367–1376
- BNF for Children
Last reviewed: 01 April 2022
Next review: 30 April 2024
Author(s): Dr A M Ewins
Version: 3
Approved By: Schiehallion Clinical Governance Group
Document Id: RHC-HAEM-ONC-020