MRSA in paediatric cystic fibrosis patients, eradication and treatment
exp date isn't null, but text field is
Disclaimer:
The following guideline has been developed for use within the Royal Hospital for Children, NHS Greater Glasgow and Clyde (NHSGGC). The guideline has been developed in collaboration with key stakeholders within NHSGGC, including Microbiology, Cystic Fibrosis, Infectious Disease and Pharmacy teams. The guideline has been approved by the Paediatric Antimicrobial Management Team and ratified by the NHSGGC Antimicrobial Utilisation Committee. The guideline does not account for epidemiology and resistance patterns outside of NHS GGC and use outside of the designated organisation is at the individual’s risk.
“MRSA infection will lead to a reduction in options for antibiotic treatment and a likelihood of deterioration in lung function, therefore MRSA infection should be avoided“
CF Trust MRSA 2008
“At first isolate, or in a person who has been free of MRSA following previous treatment, aim to eradicate the organism.“
CF Trust Antibiotic Treatment for CF 2009
“There are a small number of studies of the use of treatment regimens to eradicate MRSA lower respiratory tract infection in people with cystic fibrosis. The rate of clearance of infection without treatment is unknown. The eradication regimens in these studies have included combinations of oral, intravenous and nebulised antibiotics. The optimum regimen remains unclear...
CF Trust MRSA 2008
If one treatment regimen fails to eradicate MRSA infection, two further attempts with the same or different regimens may still be successful and should be considered.“
CF Trust MRSA 2008
CF Trust MRSA 2008:
- Every Specialist CF Centre and CF Clinic should have a microbiological surveillance and infection prevention and control policy that considers cross-infection risk for MRSA.
- The methods used and extent to which Specialist CF Centres and CF Clinics segregate patients should be determined by local policy.
- Good hygiene should be practised in all outpatient clinics and inpatient facilities to minimise the risk of transmission of MRSA between patients.
- Specialist CF Centres and CF Clinics should monitor the rate of new acquisition of MRSA.
- A policy of segregation that covers both inpatient admissions and outpatient clinics is advised.
- Stop Flucloxacillin/Erythromycin administration on first culture of MRSA
- Skin and nasal swabs - To be obtained from patient at baseline and around every inpatient admission. Anterior nares and Perineum are the minimum number of swabs required. Other sites are included if applicable, such as skin lesions/ wounds, catheter sites, e.g. Central Venous Catheters, Hickman Lines, catheter urine, umbilicus (neonates only). If patient refuses perineal screening they should be offered throat screening.
- Seek Infection Prevention and Control advice re- extended screening if there is persistent or rapidly recurrent MRSA colonisation of the patient.
- Source isolation as per local hospital policies at Out-patient and Inpatient attendances
- Sputum / Cough swab cultures obtained to monitor colonization
- MRSA would be considered eradicated if cultures remain clear for 12 months or more.
Management of MRSA infections at RHC:
The CF Team and RHC Microbiology /Infection Prevention and Control Teams have reviewed the
available information regarding current MRSA eradication and treatment regimens, including
recent local audit data. The following regimens have been selected as being most suitable for use
at RHC Glasgow CF Unit
FOR MRSA COLONISED PATIENTS NOT ON ROUTINE ANTIBIOTICS and NOT EXPERIENCING A RESPIRATORY EXACERBATION
Regime A1: MRSA COLONISATION OF RESPIRATORY TRACT +/- SKIN /NOSE COLONISATION IN PATIENTS NOT CURRENTLY RECEIVING A CFTR MODULATOR
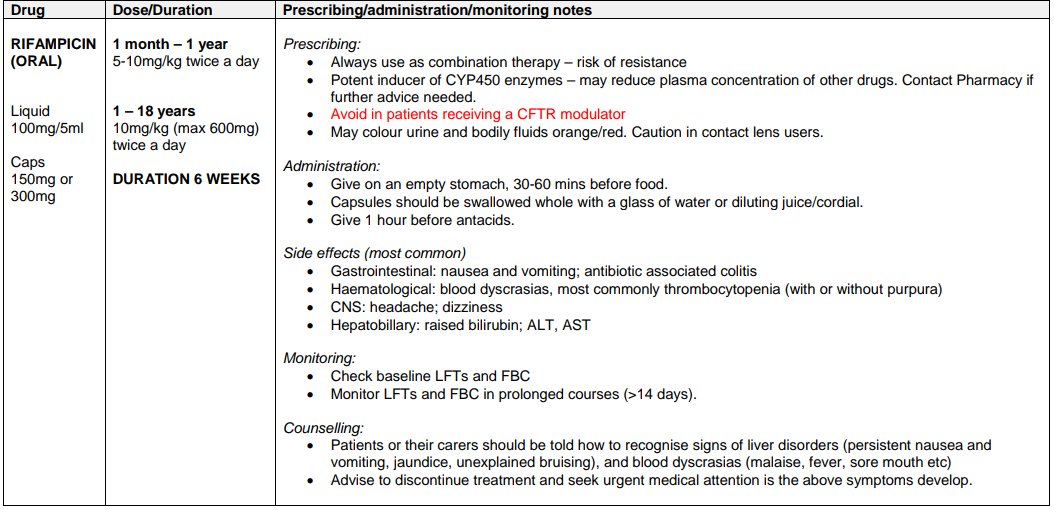
Regime A2: MRSA COLONISATION OF RESPIRATORY TRACT +/- SKIN /NOSE COLONISATION IN PATIENTS WHO ARE CURRENTLY RECEIVING A CFTR MODULATOR
Due to the risk of cytochrome mediated drug-drug interactions with the CFTR modulator therapies, patients receiving these treatments who require respiratory tract decolonisation for MRSA should be discussed with an infection specialist in the first instance.
Recommendations for decolonisation therapy will be based on individual microbiology and sensitivity patterns.
Check for drug-drug interactions before initiating treatment. For further advice please contact Pharmacy.
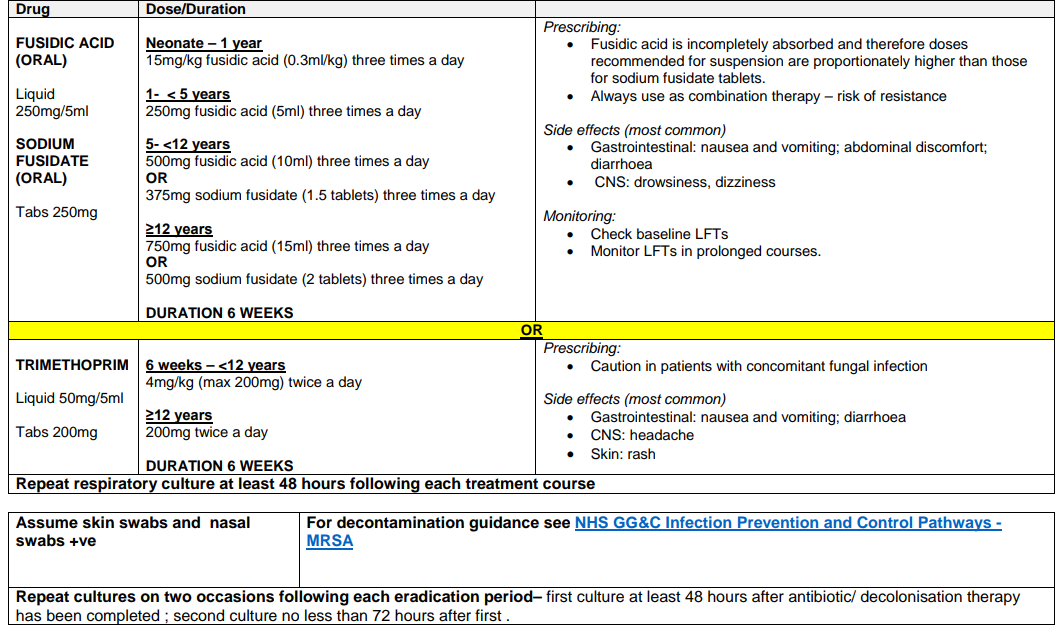
Regime B: MRSA COLONISATION OF SKIN / NOSE ONLY
| If skin swabs or nasal swabs +ve | For decontamination guidance see NHS GG&C Infection Prevention and Control pathways - MRSA |
| Repeat cultures on two occasions following each eradication period– first culture at least 48 hours after antibiotic/ decolonisation therapy has been completed ; second culture no less than 72 hours after first. | |
Eradication Regimes A and/or B should be undertaken on a maximum of three occasions
IV ANTIBIOTIC REGIMEN (MRSA COLONISATION) –RESPIRATORY EXACERBATION OR REGULAR IVs
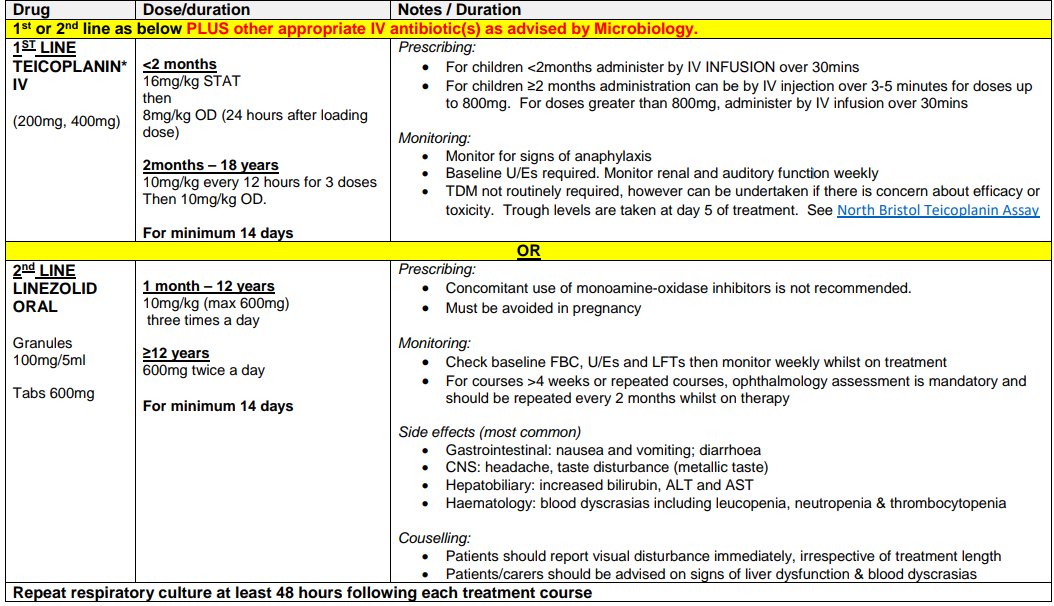
MRSA COLONISATION OF SKIN / NOSE ONLY
| Assume skin swabs and nasal swabs +ve | For decontamination guidance see NHS GG&C Infection Prevention and Control pathways - MRSA |
| Repeat cultures on two occasions following each eradication period– first culture at least 48 hours after antibiotic/ decolonisation therapy has been completed ; second culture no less than 72 hours after first. | |
MRSA - Report of the UK Cystic Fibrosis Trust Infection Prevention and Control Working Group APRIL 2008
Antibiotic Treatment for Cystic Fibrosis CF Trust 2009
Methicillin-resistant Staphylococcus aureus: impact at a national cystic fibrosis centre S.R Thomas, K.M. Gyl, H.Gaya and M.E.Hodson Journal of Hospital Infection (1998) 40: 203-209
Solis A, Brown D, Hughes J, Van Saene HK, Heaf DP. Methicillin-resistant Staphylococcus aureus in children with cystic fibrosis: An eradication protocol. Pediatr Pulmonol 2003;36:189–95.
Manufacturer’s Summary of Product Characteristics (SmPC); Rifampicin, Fucidic Acid, Sodium Fusidate, Trimethoprim, Teicoplanin, Linezolid; accessed via www.medicines.org.uk; 10/03/2021
British National Formulary for Children, App version 3.0.8 (854), updated Jan 2021.
Last reviewed: 22 February 2022
Next review: 22 February 2024
Author(s): Jane Wilkinson
Version: 3
Co-Author(s): Cystic Fibrosis Team: Dr Jane Wilkinson, Susan Kafka, Pharmacist, CF CNS Linda Gordon; FOR RHC ICP TEAM: Gillian Bowskill; Infectious Diseases/Microbiology: Dr Rosie Hague, Consultant Paediatric Immunology and Infectious Disease. Dr Christine Peters, Consultant Microbiologist
Approved By: Antimicrobial Utilisation Committee
Document Id: 636

