Neonatal transfusion guideline
exp date isn't null, but text field is
Objectives
Guidance on the appropriate use of blood and blood components for neonates
Audience
This guideline is applicable to all medical, nursing and midwifery staff working with neonatal patients in the West of Scotland. Individuals should also be familiar with the relevant pharmacy monographs. It is a requirement for all staff involved in the ordering or administration of blood components to complete, as a minimum, Module 1 of the online training package “Safe Transfusion Practice for Paediatrics” available at https://nhs.learnprouk.com/
Whilst the administration of blood and blood components can be life-saving, it is not without risk. There is a small risk of transmission of blood borne infections, there is a risk of significant morbidity if the incorrect blood component is administered and in large volume transfusions there is the potential for fluid overload and heart failure (Transfusion Associated Circulatory Overload or TACO). In the neonatal population the phenomenon of transfusion associated necrotising enterocolitis (TANEC) is a recognised association although aetiology is likely to be multifactorial. There are a number of general principles which should always be followed when administering blood or blood components.
- Only use blood components when they are clearly indicated.
- Take steps to minimise donor exposure.
- Delayed cord clamping for 1 minute (See DCC Guideline)
- Cord blood for baseline investigations in preterm <32 weeks, VLBW & ELBW babies
- Use of Paedipacks
- Take great care when checking blood components prior to administration.
- Closely monitor the patient during and immediately following administration of blood components.
Before first transfusion
- Maternal blood sample (if available)
- Maternal ABO and RhD group
- Screen for the presence of atypical red cell antibodies and identification of antibodies.
- Neonatal blood sample
- Two samples required one of which may be a cord blood sample
- Remember to take a pre-transfusion bloodspot for a baby in NICU < Day 5
- Neonatal ABO and RhD group
- Direct Antiglobulin test (DAT) on the neonatal red cells
- If no maternal sample available, screen neonate’s serum for atypical antibodies by an Indirect Antiglobulin Technique (IAT)
Before subsequent transfusions
Additional "cross-match" specimens are not routinely required for subsequent transfusions up to 4 months. They are, however, required for:
- Exchange transfusion
- All further transfusions after any large volume transfusion (50ml/kg or greater)
- Transfusions of infants > 4 months
Please note that irradiation of blood or platelets is rarely necessary and significantly reduces the shelf life of blood once performed. It also runs the risk of hyperkalaemia. There are, however, a small number of specific indications when irradiation of blood and platelets should be requested to eliminate the risk of Graft Versus Host Disease (GVHD). If you are in any doubt, seek the opinion of a senior neonatologist before requesting an irradiated product
Red cell and Platelet components should be irradiated in the following circumstances:-
- Intrauterine transfusion,
- Exchange transfusion after Intrauterine transfusion
- Top-up transfusion after Intrauterine transfusion of platelets or red cells
- Exchange transfusion in VLBW infants (<1500g), and preferable for all exchange transfusions
- Transfusion of blood components from a 1st or 2nd degree relative.
- Proven or suspected congenital, T- cell related, immunodeficiency
- Severe Combined Immune Deficiency SCID – may present with persistent (every FBC) lymphopenia <2 x 109/L
- Wiskott Aldrich Syndrome – may present with thrombocytopaenia and eczema
- 22q.11 deletion (Di George) – may present with heart defects, absent thymus, or hypocalcaemia and lymphopenia
- For Further information on the presentation of congenital immune deficiencies see the Diagnostic & Clinical Care Guidelines from the Immune Deficiency Foundation
N.B. Irradiation of blood components will be performed at the Scottish National Blood transfusion Service SNBTS. A complete pack will be irradiated and this pack will subsequently be divided into four aliquots (paedipacks) if required for small-volume transfusions.
Irradiated blood "leaks" potassium during storage, and should therefore be used within 14 days for "top-up" transfusions and within 24 hours for exchange transfusions. In cases of established hyperkalaemia a fresh unit of blood should be requested if the existing pack has previously been irradiated.
In view of the significantly reduced shelf life it is important to avoid unnecessary irradiation of blood components as this contributes to blood wastage and increases donor exposure.
If blood is required in an emergency, e.g. blood loss at delivery, O RhD -ve blood should be used. A blood grouping sample and Newborn Bloodspot should be taken from the baby prior to emergency transfusion
1 unit (In some hospitals this may be divided into 4 Paedipacks) of O RhD -ve, leucodepleted, CMV and Hepatitis E -ve blood is available for emergency paediatric use in the satellite blood fridge, the locations are site specific:
|
Local Arrangements PRM – In the corridor outside the obstetric theatres RHC – In the blood gas room in labour ward RAH – In Labour ward |
Where Bloodtrack is used the blood will need to be collected by a staff member trained in the Bloodtrack system. A member of labour ward staff may be asked to collect the blood for you if required in these circumstances. The emergency button is pressed, the fridge will open & the unit can be removed & scanned. The pressing of the emergency red button will also alert Blood Bank that the fridge has been opened.
Where Bloodtrack is not used staff should use local procedures for the retrieval of emergency blood packs
Take care to avoid taking units for emergency adult use or specific patients
The paediatric packs will be labelled as follows:
FOR PAEDIATRIC USE ONLY
UNMATCHED BLOOD - FOR EMERGENCY USE ONLY
DO NOT INFUSE PRIOR TO OBTAINING A BLOOD SAMPLE FROM THE BABY
FILL IN THE PATIENT NAME, DATE OF BIRTH AND HOSPITAL NUMBER ON THE BLUE TRACEABILITY LABEL AS SOON AS POSSIBLE
INFORM BLOOD BANK
In PRM the paedipack also has a teddy bear tag to facilitate distinguishing it from packs for adult use.
After use
The pack label should be placed in the neonatal patient’s medical record.
The blue traceability label should be filled in and returned to Blood Bank.
Blood bank should be informed to allow prompt replacement.
NB - On sites where the emergency blood is divided into paedipacks, the unused packs may be retained by Blood Bank should the neonate require further transfusions.
If the required volume for infusion is large a further cross match sample may be required.
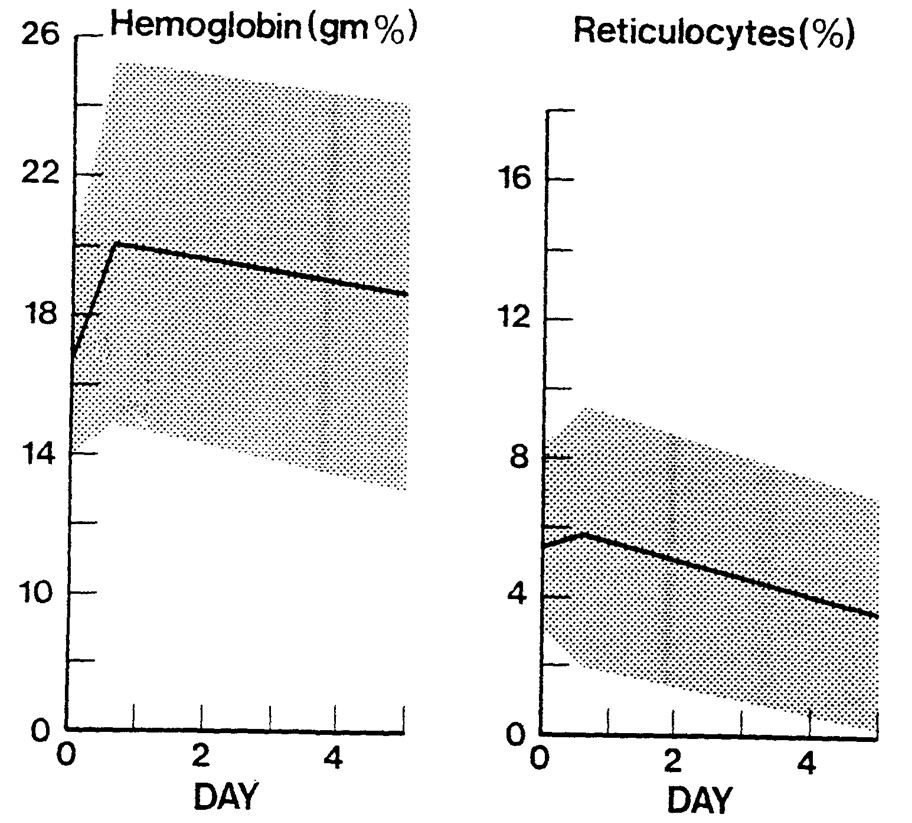
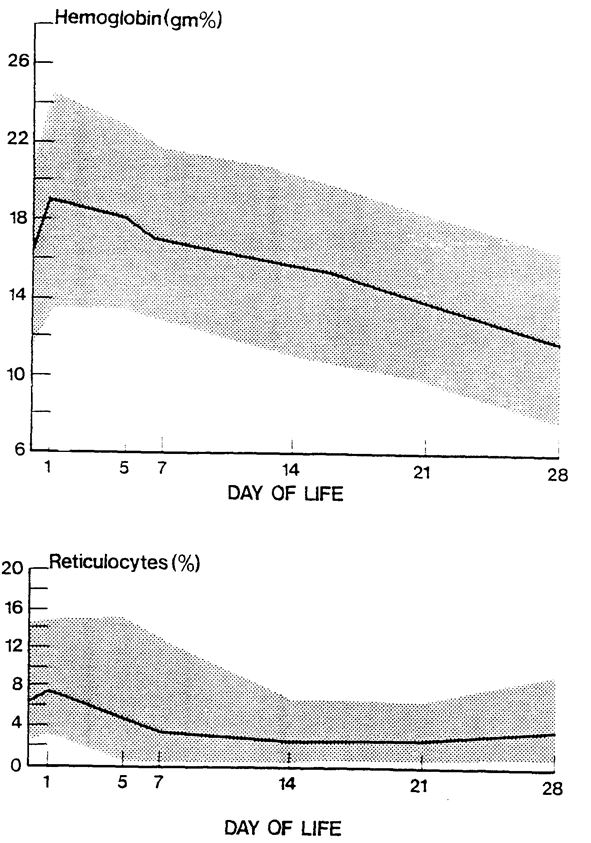
Anaemia in the neonatal patient is frequently due to a combination of blood loss due to phlebotomy and inefficient erythropoiesis, although a smaller number of patients will have anaemia due to other causes of blood loss. The need for transfusions increases inversely with the size and gestation of the patient due to the smaller circulating blood volumes. Despite efforts to minimise the need for transfusion, most babies under 28 weeks will have a degree of anaemia and some will require transfusion.
Reducing the need for blood transfusions.
- Delayed cord clamping: Delayed cord clamping reduces the need for subsequent blood transfusion along with other benefits. Staff should refer to the WoS Guideline “Delayed Cord Clamping” for further information.
- Avoid unnecessary phlebotomy losses: Please take note of the investigation guidelines and the blood sampling guidelines in your local unit to ensure that only essential investigations are performed and the minimum volume of blood required for the tests is withdrawn from each patient. Consider whether an investigation is required or whether trends can be monitored on the blood gases (eg Na/Hct)
- Use of cord blood for initial blood tests for VLBW neonates has been advocated in order to reduce the need for transfusion, but results should be interpreted with caution if there are sampling difficulties.
- Conservative transfusion practice: Conservative transfusion practices have been shown to be well tolerated and to reduce transfusion events and donor exposures. The following transfusion triggers/thresholds are designed to encourage conservative transfusion practices and are guidelines only. Some very sick/septic infants will require transfusion at higher Hb concentrations in order to optimise tissue oxygen delivery; such infants require discussion with a consultant.
- There is no indication for transfusion to replace phlebotomy losses alone.
Transfusion Triggers for preterm neonates <32 weeks (From BCSH Guideline)
Note – these thresholds are for guidance only, and are intended to encourage restrictive transfusion practices. Transfusion decisions should be taken on an individual clinical basis.
|
Postnatal Age |
Suggested Transfusion threshold Hb (g/l) |
||
|
Ventilated |
On Oxygen, CPAP or High Flow |
Off Oxygen and respiratory support |
|
|
Day 1 (1st 24h) |
<120 |
<120 |
<100 |
|
Day 2-7 (1st Week) |
<120 |
<100 |
<100 |
|
Day 8-14 (2nd Week) |
<100 |
<95 |
<75 |
|
Day15 Onwards |
<100 |
<85 |
<75 |
Unit characteristics:
- Packed cells are supplied in additive solution (SAG-M red cells) with a haematocrit of 0.5–0.7.
- Where possible, Paedipacks (70ml aliquots) should be used allowing up to 4 transfusions from a single donation. Paedipacks from the same unit may be given for the shelf life of 35 days however this is reduced to 14 days if irradiated.
- All units selected for neonatal use are CMV and Hepatitis E negative
- All red cell products for babies are leucodepleted
- Small Volume transfusion: For neonatal patients this must be calculated in mls/kg and prescribed as the number of mls to be infused.
Transfusion volumes of 15 ml/kg are generally recommended for non‐bleeding neonates (up to 20mls/kg)
- Larger volumes or repeated transfusions may be required in some patients. Due to high risks of morbidity in such infants, including the risks of fluid volume overload from large volume transfusions, discussion with the neonatal consultant is required
- Ongoing blood loss
- Disseminated Intravascular Coagulation
Irradiation - NB this is infrequently required
Please see advice on irradiation – if in doubt consult a senior neonatologist before requesting irradiated packed cells as this will reduce the shelf life of the remaining paedipacks and increase the risk of additional donor exposure as well as contributing to avoidable blood wastage.
Administration:
- The blood must be administered within 4 hours of removal from the fridge. It is therefore important to ensure the patient has a working cannula available for the transfusion before requesting blood to avoid unnecessary delays.
- There is no need for routine furosemide cover for small volume transfusions unless there are existing signs of heart failure.
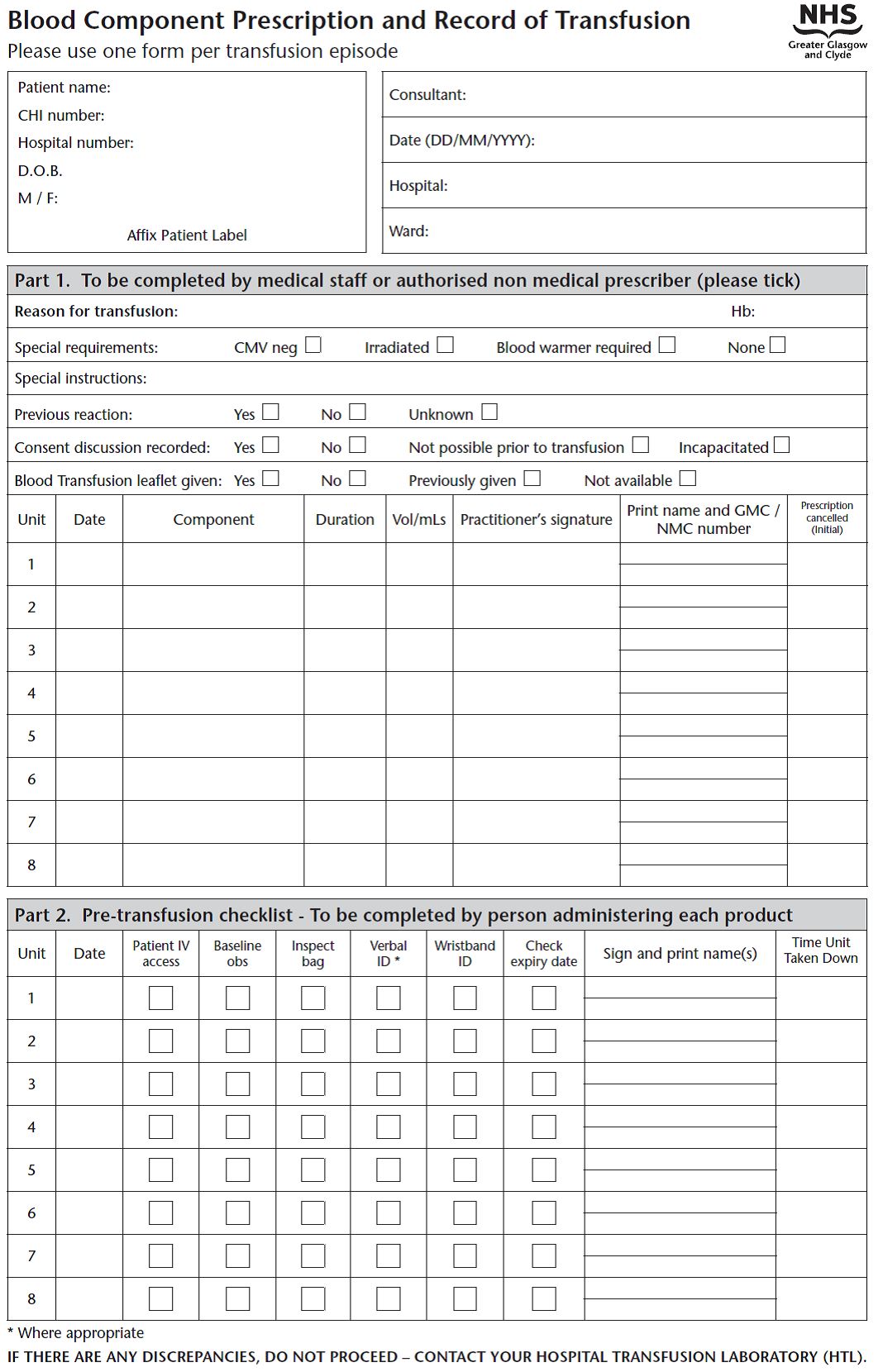
- The practitioner must authorise the total volume in millilitres and the duration of blood transfusion on a Blood Component Prescription Chart (see appendix)
- Patient and blood component details must be checked at the cot side, crosschecking the patient details with the baby’s name band. (patient name, hospital number, blood component unit number, blood group and expiry date)
- The sticker from the blood component must be placed on the Blood Component Prescription Chart, the volume administered recorded & signed & the chart should be filed in the infant’s casenotes.
- Observations (temperature, Heart Rate and Respiratory Rate) must be recorded during the infusion.
- The remainder of the component must be kept until the infusion is complete in case of a transfusion reaction.
- The blue traceability label should be completed with patient details & returned to blood bank. This is audited monthly and is essential to maintain quality in the blood transfusion process
- Note – some departments advocate the cessation of feeds for the duration of the transfusion in the belief that this may reduce the incidence of TANEC. The evidence is not robust and the practice does not form part of this guidance. Please refer to local policy.
Transfusion and necrotising enterocolitis (NEC):
Infants with NEC may occasionally be systemically infected with neuraminidase-producing organisms, such as the Clostridium species. Neuraminidase can strip residues from red cells exposing the T-antigen and increasing the risk of haemolysis. This is known as "T-antigen activation". There is currently no evidence to support routine testing on infants suspected of NEC. National guidelines based on the best evidence suggest screening only for T activation in an infant who develops haemolysis following a blood component transfusion, and as part of a haemolysis screen (i.e. Coombs test, blood film, reticulocyte count, serum bilirubin, as well as G6PD and pyruvate kinase concentrations-liaise with haematology and see separate guideline). If transfusion is required for neonates with T‐activation (usually in the context of NEC) and haemolysis following previous transfusion, red cells in SAGM are suitable as these contain little plasma.
Transfusion Triggers:
Exchange transfusion is required in infants with severe Haemolytic Disease due to blood group incompatibilities or G6PD deficiency. It is used in these infants to manage severe anaemia at birth Hb <100 g/l, or to treat severe hyperbilirubinaemia which is not controlled by phototherapy and, where appropriate, intravenous immunoglobulin therapy.
The decision to carry out an exchange transfusion will be taken at consultant level but is usually required if the bilirubin exceeds, or is anticipated to exceed, the exchange transfusion threshold on a gestation appropriate phototherapy chart – see separate guideline
Unit Characteristics:
Plasma-reduced ("partially packed") red cells with a haematocrit of 0.5-0.6 are suitable for ET for both hyper-bilirubinaemia and severe anaemia, this should be the only component used for ET. These units should be less than 5 days old. Irradiation is required for VLBW babies and advised for term babies.
Sourcing and cross-matching appropriate units may take some time and requirements must be discussed with blood bank as soon as ET is anticipated. In the case of known, severe haemolysis in-utero, blood will need to be ordered before delivery ideally giving Blood Bank 24 hours of notice. This requires liaison with the obstetric team and Blood Bank in advance of the expected delivery date/time - see separate guidance for the management of the mother with irregular antibodies. If a situation arises unexpectedly whereby an ET is indicated if the mother’s group and antibodies are known, the specialised pack is relatively easy to source from Edinburgh and with the added time for irradiation can be delivered in 3-4 hours. If the mother has rare antibodies then the whole process may take longer, potentially up to eight hours. If the clinical need is more urgent then a discussion between the consultant neonatologist and haematologist should take place to decide whether an alternative suitable unit which is more readily available can be used instead.
Volume to be exchanged: Twice the circulating blood volume (80ml x 2 = 160ml/kg). Using a double-volume exchange is more effective at removing maternal antibody from the baby’s circulation although even this volume does not remove all the antibody.
Technique: ET may be performed either via a single umbilical venous line or by using a UAC and a UVC combined (blood is withdrawn using the arterial line and replaced via the venous line). Umbilical lines should be placed and position confirmed by x-ray as per WoS policy.
- ET is a sterile procedure.
- Pre ET check FBC, Coagulation, U+E, Ca, Glucose, SBR and blood gas.
- A blood warmer should be used.
- Each aliquot should be approximately 8ml/kg, indeed initial aliquots may be of smaller volume until it is clear the procedure is well tolerated.
- Each aliquot should be removed and replaced over a 5 minutes cycle. Always commence an ET by removing an aliquot of blood. Each aliquot removed and replaced should be timed by the neonatal nurse assisting with ET and recorded on a standard ET recording chart (see appendix).
- The ET should take ~2 hours (longer if poorly tolerated and smaller aliquots are required).
- Blood glucose monitoring is required after every 4 cycles.
Post Transfusion management:
- Repeat the bloods taken prior to the exchange - FBC, Coagulation, U+E, Ca, Glucose, SBR & gas.
- Continue phototherapy
- Continue to monitor serum bilirubin concentrations 4 hourly as a rebound hyperbilirubinaemia may occur.
- While these babies have evidence of haemolysis they should receive folic acid supplementation
Immunoglobulin therapy for Alloimmune Haemolysis
A Cochrane review by Alcock et al. in 2009, indicated that significantly fewer infants treated with intravenous immunoglobulin (IVIG) required Exchange Transfusion (ET), relative risk 0.28 (CI 0.17-0.47). The number needed to treat in order to prevent one ET was 2.7 (CI 2.0-3.8). This result was based on a relatively small number of patients but goes some way to support the use of IVIG in infants with Alloimmune Haemolytic Disease of the Newborn (HDN) in whom the Serum Bilirubin (SBR) continues to rise despite appropriately administered phototherapy – see separate guidelines for the management of jaundice.
Dose: 500mg/kg Intravenous Immunoglobulin given by infusion over 4h. The dose may be repeated once after 48h if required.
Supply: Immunoglobulin is ordered via pharmacy using an Immunoglobulin request form – see appendix of the pharmacy monograph for Immunoglobulin.
Emergency Supplies: A small number of units are kept locally within the neonatal units and in the emergency drug cupboards. See local details below
|
Local Arrangements PRM
RHC
RAH
|
Disorders of coagulation are common in the neonatal intensive care setting.
Very immature infants have low levels of most clotting factors and may have prolonged clotting times. However, correction of these prolonged clotting times using FFP, in the absence of clinically significant bleeding, has not been shown to reduce the risk of IVH. Therefore, routine estimation of coagulation in otherwise well preterm infants is not recommended.
Other infants have acquired coagulopathies secondary to sepsis, NEC or asphyxia.
NB See separate advice for inherited disorders of coagulation in the Haematology Guideline.
When to perform a coagulation screen:
Coagulation screens should be performed in the following situations.
- Hypoxic Ischaemic Encephalopathy (HIE) – See Cooling Guideline
- Pre exchange transfusion – see Exchange Transfusion Section above
- Clinical indications e.g.
- Bruising/bleeding tendencies
- Thrombocytopenia
- Sepsis / NEC
NB – Coagulation results will be unreliable if samples are taken from a heparinised line even if a large volume of dead space has been aspirated. Samples should be taken by Venepuncture or from a line, which has not been flushed with heparin.
BSH guidelines recommend that FFP should be used for:
- Clinically significant bleeding
- Prior to invasive procedure with a risk of significant bleeding
- Abnormal coagulation profile
- Neonatal Purpura Fulminans; pending confirmation of diagnosis of protein C or Protein S deficiency and in the case of Protein C deficiency the availability of Protein C concentrate
FFP should not be used as routine prophylaxis against peri/intraventricular haemorrhage in preterm neonates, as a volume replacement solution, or just to correct “abnormalities” of the coagulation screen.
Transfusion triggers
- All neonates in the above groups – APTT > 75
Unit Characteristics
- A single unit of FFP will contain around 230 ml
- FFP is subjected to virus inactivation using photo-inactivation with white light in the presence of methylene blue (MB FFP).
- CMV negative units are usually selected for use in neonates. However as FFP is leucodepleted, CMV positive units may be used if there are no available CMV –ve units.
Volume required: 15 – 20 ml/kg FFP over 30 minutes
N.B. Platelet packs have a large coagulation factor component. If platelets and FFP are both required, BTS recommend giving platelets first, at a slightly increased dose (20ml/kg), then reassessing coagulation before using FFP. This may minimise volume overload (by negating the need for FFP)
Repeat coagulation screen following administration of FFP to assess further need for blood components
Transfusion triggers:
Cryoprecipitate is prepared from a unit of FFP and has a higher concentration of FVIII, VWF, FXIII, fibronectin and fibrinogen than FFP. It is not a straightforward, more concentrated, alternative to FFP. It is therefore used for infants with severe coagulopathy with low fibrinogen levels(<1 g/L) despite administration of platelets and / or FFP.
Approval is required from on-call haematologist.
Unit Characteristics:
- A pack contains about 20-40ml
Volume for transfusion:
- Stable patient – 5 – 10 ml/kg over 30 mins.
FFP and cryoprecipitate can be given in the same line if required. - Acute haemorrhage – where haemorrhage is not controlled using standard doses, additional aliquots of FFP and Cryoprecipitate may be given under the direction of a consultant neonatologist
Thrombocytopenia is common in the neonatal setting affecting as many as 22% of NICU admissions in one study. Causes of thrombocytopenia can be classified into disorders of increased destruction, increased consumption, or decreased production. Despite its frequency, historically there has been very little evidence from which to gauge optimal management in neonatal patients although some evidence is beginning to emerge.
NB – Platelet clumping in a FBC sample can give a false diagnosis of thrombocytopaenia especially when taken from a poorly flowing capillary specimen. This is often identified by the laboratory but if there remains any doubt a further sample should be taken to confirm the diagnosis before treatment is commenced.
Transfusion triggers:
- All Patients - Platelet count < 25 x 109/l
Stable platelet counts above 25 x 109/l in an otherwise well patient are usually well tolerated and are not associated with spontaneous haemorrhage. These patients require regular monitoring of the platelet count. THIS INCLUDES NEONATES WITH NO BLEEDING (ALSO INCLUDES NEONATES WITH NAIT IF NO BLEEDING AND NO FAMILY HISTORY OF INTRACRANIAL HAEMORRHAGE) - Patients with additional risk factors – Platelet count < 50 x 109/l
- Active bleeding. NB. unless the diagnosis is known, investigation is required to rule out other disorders of coagulation, especially Disseminated Intravascular Coagulation (DIC). Send a sample for coagulation prior to platelet transfusion.
- Before invasive procedures e.g. LP, SPA or insertion of central line
- Acute thrombocytopenia of any cause
- INFANTS WITH NAIT IF PREVIOUSLY AFFECTED SIBLING WITH INTRANCRANIAL HAEMORRHAGE
- Patients with major bleeding or requiring major surgery g. neurosurgery – platelet count <100
Units characteristics:
- Platelets are supplied as CMV and hepatitis E negative packs
- Platelet packs are leucocyte depleted by filtration
- Platelets are supplied which are free of clinically significant irregular antibodies
- Labelled ‘platelets for neonatal use’
- There are two types of pack available for use
- Pooled donor packs
- Pooled donor packs are prepared from 4 blood donations
- The volume of each pack will be approximately 310ml
- Apheresis packs
- Apheresis units are prepared from a single donor and are the preferred type of unit, when available, for this reason.
- The packs are of larger volume and may be separated into 4 smaller paediatric units if repeated transfusions of the same patient are anticipated.
Volume for transfusion: For neonatal patients this must be calculated in mls/kg and prescribed as the number of mls to be infused.
- Stable infant – 15 – 20 ml/kg over 30 minutes
- Additional risk factors – Larger volumes or repeated transfusions may be required in the following infants. Due to high risks of morbidity in such infants, including the risks of fluid volume overload from large volume transfusions, such patient require discussion with the neonatal consultant
- Active bleeding
- DIC
- Severe Neonatal Alloimmune Thrombocytopaenia
Irradiation: NB. This is infrequently required
Indications for irradiation of platelets are the same as for red cells – refer to advice above
Administration:
- If blood and platelets are required, platelets should be given first
platelet transfusion is quicker
This may help control ongoing bleeding
- Platelets should be administered shortly after receipt to avoid clumping which reduces efficacy
- Platelets should be drawn up through a blood transfusion giving set to ensure the removal of aggregates.
Post Transfusion Management:
- It is usually necessary to perform a post-transfusion FBC to asses the increment in the platelet count.
- If there is evidence of ongoing platelet consumption a further FBC will be necessary to assess the ongoing rate of fall of the platelet count. The timing of this interval will depend on the severity of the thrombocytopaenia.
- Persistent or refractory thrombocytopaenia requires investigation of the cause if not already known. Causes include sepsis, DIC, and Neonatal Alloimmune Thrombocytopaenia (NAIT)
Neonatal alloimmune thrombocytopenia (NAIT) occurs when fetal platelets contain an antigen inherited from the father that the mother lacks (akin to rhesus incompatibility). The mother forms IgG class antiplatelet antibodies against the "foreign" antigen; these cross the placenta and destroy fetal platelets, resulting in fetal and neonatal thrombocytopenia. NAIT often develops in the first pregnancy of an at-risk couple but as with Rhesus disease does increase in severity in subsequent pregnancies. There are a number of antigens however Human Platelet Antigens (HPA) -1a and HPA–5b are responsible for 95% of cases of NAIT in caucasians. NAIT can result in severe thrombocytopenia. The most serious complication is intracranial haemorrhage, which occurs in 10 to 20% of affected newborns. Of these up to 50% occur in-utero. All neonates with NAIT (or suspected NAIT) and thrombocytopenia after birth should be discussed with a haematologist.
Clinical features: Incidence of NAIT has been estimated at 1 in 1000 to 5000 births. Affected newborns are otherwise healthy, with signs consistent with thrombocytopenia; widespread petechiae and bruising, with haematoma formation around the site of vitamin K administration. In many cases NAIT may be diagnosed following the incidental finding of a reduced platelet count. There is a significant risk of bleeding (more so than in thrombocytopenia associated with maternal thrombocytopenia)
Investigation:
- FBC, film and coagulation
- Screen for CMV
- Review for possible sepsis, Thrombocytopaenia is a non red flag clinical indicator for sepsis (see Early Onset Sepsis MCN guideline)
- Maternal platelet count
- Maternal platelet antigens & antiplatelet antibody (ask O&G to do next working day, sent in EDTA bottles)
- In some cases it may be necessary to determine paternal platelet antigens also
- Cranial USS
Management:
-
< 25 x109 in the absence of bleeding
OR
<50 X109 if previous affected sibling with Intracranial haemorrhage (give 15ml/kg platelets) using, in order of preference:
- Matched platelets, these may take time to arrange - liaise with BTS as soon as the diagnosis is suspected.
- Human Platelet antigen (HPA)–1a negative HPA-5b negative platelets. These are likely to be effective in ~95% of cases where the antibody types are not yet known.
- Washed platelet concentrate from the mother. These donations must be irradiated before use.
- If HPA‐1a/5b‐negative platelets are unavailable or ineffective in producing a platelet rise, random donor platelets and/or IVIg may be used, which may reduce the need for platelet transfusions until spontaneous recovery in platelet count occurs 1–6 weeks after birth.
- A post‐transfusion platelet count should be measured to check the increment.
- Use Intravenous Immunoglobulin - 1gram/kg per day for two days is effective however the response may be delayed for 24-48h. However, this must be used as an adjunctive therapy to platelet transfusion. If random donor platelets are used, BTS suggest an increased dose of 2g/kg immunoglobulin concurrent with platelets. Order from Pharmacy and prescribe as a 4 hour infusion. Routine blood observations are required during infusion.
- Liaise with obstetric staff. - There are implications for future pregnancies (recurrence is more than 75%) management may include maternal immunoglobulin therapy during pregnancy and the avoidance of assisted delivery. Referral to a fetal medicine unit is often required and antenatal therapy given. A copy of the discharge letter should be sent to the Consultant obstetrician.
Indication: Treatment for severe neutropenia - consultant approval required.
NB –Routine use of G-CSF or GM-CSF for the treatment of neutropaenia in neonates has not been shown to reduce the incidence of sepsis or improve outcomes in established sepsis. There may be a role in certain rare conditions of congenital neutropenia; liaise with haematology prior to use
Dose: Preparation (and dose) may vary between units – refer to local pharmacy monographs / data sheets.
Discontinue treatment if WCC exceeds 20 x 109/litre
Indication: Consider in symptomatic hypoalbuminaemia - albumin < 20g/l
N.B. This is a pooled blood component with a consequent risk of blood borne infection. Consider benefit carefully as it is unlikely to improve serum albumin numbers! Instead consider the aetiology of the hypoalbuminaemia.
Dose: 1g/kg = 5ml/kg/dose. Infuse over one hour
Supply: Order from Pharmacy
There is now accepted adult and paediatric evidence that there is no benefit obtained from the use of 4.5% albumin as a resuscitation fluid. Indeed much evidence suggests that there is a worse outcome if 4.5% albumin is used compared to crystalloid solutions such as 0.9% sodium chloride. Therefore in WoS neonatal units 0.9% saline is the fluid of choice for newborn resuscitation.
Serious Hazards of transfusions (SHOT)/ Serious Adverse Blood Reactions & Events (SABRE) continue to occur and paediatric patients as a group are over-represented. The risks of such events can be minimised by following good clinical practice which includes having a guideline, good documentation & communication & also giving consideration to safety aspects e.g. Avoid routine top ups out of hours and ensure the component is prescribed in mls
Communication
- Parents
- Informed consent should be taken except in the case of an emergency – There is currently no requirement for written consent however the conversation should be recorded in the casenotes.
- For premature / sick infants admitted to NICU, where the likelihood of needing blood components on one or more occasions is high, a single instance of informed consent is required at the beginning of the patient’s clinical course.
- For low risk infants where there was no anticipation of the need for blood components consent should be sought at the time of transfusion, by telephone if necessary. However this should not delay the administration of blood, or blood components, in an emergency situation.
- Parents should be offered the leaflet
“Babies receiving blood transfusion. Information for parent and carers” prior to transfusion where possible and given the opportunity to ask questions. For preterm babies in ITU babies where the likelihood of transfusion is higher, the leaflet can form part of the Admission Pack
- Informed consent should be taken except in the case of an emergency – There is currently no requirement for written consent however the conversation should be recorded in the casenotes.
- Blood bank – when calling blood bank the following information should be conveyed clearly:
- Blood component required
- Any special requirements eg irradiated (NB irradiation is seldom required)
- The degree of urgency
- The location for delivery of the blood
- Colleagues
- Medical handover – Ensure the new team understand the indication for the transfusion and what further investigations are required once the transfusion is complete.
- Nursing staff – Ensure the new team understand the indication for the transfusion and what observations are required.
- If consent could not be obtained prior to an emergency transfusion the new team should inform the parents and give them the transfusion leaflet at the next contact with the parents
Documentation
- Record the details of the discussion with the parents, confirming that they have given their consent. You should also confirm that this has occurred on the Blood Component Prescription Chart (tick box).
- Record details of the indications for transfusion of the blood components. This will include the current haematology Laboratory results.
A record of the clinical indications for transfusion is particularly important where a decision has been made to transfuse at levels other than the “transfusion triggers” in the sections on the individual components earlier in this document. - Record details of the component transfused and the volume given
- Where appropriate (see above), Record the response to transfusion – Change in laboratory values or clinical response
- Documentation of indication for transfusion (eg Hb, clinical parameters)
- Transfusion appropriate (adheres to guideline or if deviates, clinical indicators documented)
- Parents given information leaflet
- Documentation of communication with parents including agreement (not necessary for transfusion in the emergency setting)
- Transfusion prescribed in millilitres
- Documentation of transfusion
- Documentation of monitoring during and after transfusion
- Documentation of assessment of patient post transfusion (eg Hb, colour, clinical parameters)
- For small volume transfusions – takes place “in hours” as opposed to “out of hours”
- For Exchange Transfusion – written consent (BAPM recommendation)
|
Patient’s ABO group |
ABO group of Blood Products to be Transfused |
|||
|
Red cells |
Platelets |
FFP |
Cryoprecipitate |
|
|
O |
|
|
|
|
|
First Choice |
O |
O |
O* |
O* |
|
Second Choice |
- |
A or B or AB |
A or B or AB |
A or B or AB |
|
|
|
|
|
|
|
A |
|
|
|
|
|
First Choice |
A |
A |
A |
A |
|
Second Choice |
O |
AB |
AB |
AB |
|
Third Choice |
- |
B† |
B¥ |
B¥ |
|
Fourth Choice |
- |
O† |
- |
- |
|
|
|
|
|
|
|
B |
|
|
|
|
|
First Choice |
B |
B |
B |
B |
|
Second Choice |
O |
AB |
AB |
AB |
|
Third Choice |
- |
A† |
A¥ |
A¥ |
|
Fourth Choice |
- |
O† |
- |
- |
|
|
|
|
|
|
|
AB |
|
|
|
|
|
First Choice |
AB |
AB |
AB |
AB |
|
Second Choice |
A or B |
A† |
A¥ |
A¥ |
|
Third Choice |
O |
B† |
B¥ |
B¥ |
|
Fourth Choice |
- |
O† |
- |
- |
|
|
|
|
|
|
|
Unknown |
|
|
|
|
|
First Choice |
|
AB |
AB |
AB |
|
Second Choice |
|
A† |
A¥ |
A¥ |
|
Third Choice |
|
B† |
B¥ |
B¥ |
|
Fourth Choice |
|
O† |
- |
- |
Platelets - † Tested and negative for HT antibodies: here denoted on the component label this indicates that the component has been tested and contains a low titre of anti-A or anti-B in the plasma.
- Group B or AB platelets may not be available. However, the use of group O platelets for non-O patients should be avoided as much as possible. Platelets should be compatible for D.
- If a patient requires HLA-matched platelets, HLA match usually takes precedence over ABO group
FFP & cryoprecipitate:
* Group O FFP and cryoprecipitate should only be given to group O patients.
¥ Group compatible plasma should be used wherever possible. MB FFP, SD FFP and MB cryoprecipitate are not tested for HT antibodies. Non-compatible groups should only be used in emergencies when compatible groups are not available.
- AB plasma, though haemolysin free and suitable for patients of any ABO group, should be conserved for group AB patients or emergency transfusions where the patient’s groups is unknown. Group AB MB cryoprecipitate has limited availability
Transfusion for foetuses, neonates and older children, Br J Haematol. 2016; 175: 784-828 New etal PLUS Addendum August 2020
National Comparative Audit for Blood Transfusion 2010
Curley, A., Venkatesh, V., Stanworth, S., Clarke, P., Watts, T., New, H., Willoughby, K., Khan, R., Muthukumar, P. & Deary, A. (2014) Platelets for neonatal transfusion - study 2: a randomised controlled trial to compare two different platelet count thresholds for prophylactic platelet transfusion to preterm neonates. Neonatology, 106, 102– 106.
Last reviewed: 24 November 2021
Next review: 23 December 2024
Author(s): Dr K Kasem – Consultant Neonatologist PRM
Co-Author(s): Other Professionals Consulted: Dr Louisa McIlwaine – Consultant Haematologist; Moira Caldwell – Transfusion Practitioner; Stephen Bowhay – Pharmacist
Approved By: West of Scotland Neonatology Managed Clinical Network

