For all pain enquires, APRS team are available Monday to Friday 0800-1600 on Ex 84319. Outwith these hours please contact the resident on call anaesthetist on Ex 84342.
Fentanyl NCA / PCA pilot - Ward 3c (Renal)

Objectives
To provide a clear guidance for the safe pilot use of Fentanyl by Patient Controlled Analgesia (PCA) or by Nurse Controlled Analgesia (NCA) for a specific sub group of patients, nursed on ward 3A Renal.
Scope
The pilot aims to cover patients nursed on 3C with strict inclusion and exclusion criteria, detailed below. These include, but are not limited to, children with significant renal impairment, and/or children who are post renal transplant who require acute or post-operative pain management. The prescription and PCA/NCA pump settings follow a comprehensive review of protocols from other paediatric centres, and have been agreed by a collaborative multi-disciplinary steering group with input from paediatric nephrology, palliative care, pharmacy and pain management. It is anticipated that expansion to other patient groups and/or locations within the Royal Hospital for Children will be undertaken following conclusion of the pilot subject to associated review processes and governance.
Audience
This protocol is intended for use by medical, nursing and allied health professionals involved in the care of patients with renal impairment and/or post renal transplant, who meet the criteria below and who require analgesia as part of their post-operative care or acute painful condition. Programming of the analgesic devices should only be undertaken by pain nurses, anaesthetists or appropriately trained staff.
Fentanyl PCA/NCA is used extensively across other tertiary paediatric centres. The pain team also have experience of using it safely both in other centres, and in RHC for individualised cases. We therefore have a background wealth of experience of its safe use in the paediatric population. It is also used across the adult site (QEUH) which provides an additional level of safety for rotating anaesthetic trainees who will be familiar with its use. Moreover, the use of Fentanyl as a primary agent in specific patient groups (for example patients with renal Impairment) would be recommended as best practice given its pharmacokinetics and pharmacodynamics properties.
- All patients prescribed PCA/NCA opiates should have at least PRN anti-emetics prescribed. As per APRS protocol, first line anti-emetic is Ondansetron 0.15milligrams/kg up to 8 hourly.
- Oxygen may be prescribed as required
- Naloxone – as per APRS, recommendations are :
- If a child on an opiate infusion is excessively sleepy, or with a RR <10 (<20 for infants), the infusion should be stopped and the ward/parent team should be called to review.
- As part of the standard ABC assessment of an unwell child, naloxone should be administered.
- For Severe (life threatening) respiratory depression = 400micrograms
- For sedation/mild respiratory depression/itch = 1microgram/kg
- This can be repeated at 5 minute intervals for 5 doses, maximum dose 2mg. Ideally given IV but can be given IM or SC if these routes are available. By IM and SC routes, both the dose and the frequency are the same as above.
- Consider smaller dosing initially in children at risk of withdrawal, or in children who will continue to need opiates (post-operative care, palliative care, mucositis, sickle cell crisis.
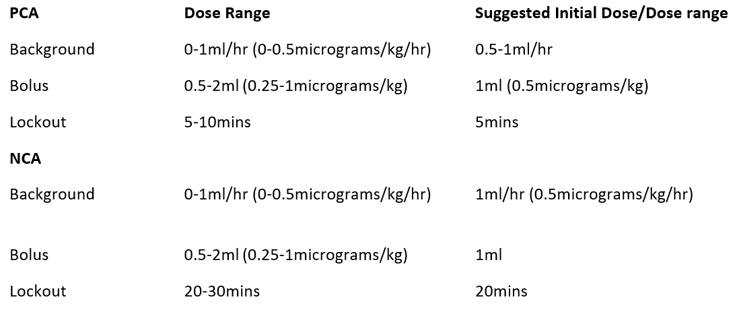
Following initial patient data (February –October 2025, 15 patients), All patients should receive a fentanyl loading dose prior to starting a fentanyl PCA/NCA.
- Dose guidance:
- Minimum 1 microgram/kg before initiating PCA/NCA, up to 50microgram/dose (>50kg)
- For renal transplant or major urological surgery, up to 5 microgram/kg, titrated to effect.
- Maximum dose at the discretion of the anaesthetist
- All patients should be fully monitored throughout
- Factors to Consider:
- Operation type / indication
- Age
- Weight
- Renal function
- Suggested loading protocol :
- Use prepared PCA/NCA syringe (concentration 0.5 microgram/kg/ml)
- Decant 5 ml into a syringe
- Administer 1 ml at a time (0.5microgram/kg), titrated to effect
In addition to increasing continuous (background) rate, patients should be routinely reloaded with up to 2microgram/kg in divided doses, titrated to effect. If reloading through the pump, this would equate to 4ml, in 1ml aliquots (1ml=0.5microgram/kg). As above, patients should have continuous monitoring during reloading.
Patients on NCA/PCA Fentanyl should:
- Be attached with an anti-siphon (non-return) valve
- Be nursed in a central location within 3C
- Be centrally monitored, or as a minimum hourly obs with continuous Saturation monitoring
- Nursed for their initial post-operative period with a nursing ratio of at least 1:2. This is already in place both on PICU (day 0 post op for renal transplant patients) and on 3C where they have 1:1 nursing care for 4 days/nights thereafter.
- Be reviewed at least daily by the APRS
- Have syringe changes by APRS or Anaesthetics on call (84342) only
- Prior to pilot, there will be a package of training aimed at nursing staff, facilitated by the Acute Pain Service and Leanne Millar (nurse educator 3C).
- This will include targeted training through learnpro modules, and refresher training on PCA safety, common side effects and trouble shooting.
- The use of fentanyl PCA/NCA will be included as part of the Acute Pain induction material, delivered to new nursing staff and rotating anaesthetic trainees.
- Designation of pain link nurses for ward 3C (possibly by way of secondment to pain service, returning back to 3C). These nurses will be :
- empowered to take an active role in each patient’s pain management, learning about the pharmacodynamics and pharmacokinetics of each drug, effects, side effects and trouble shooting
- supported by the acute pain team to be more involved in escalating and de-escalating of pain management techniques including weaning to oral opiates.
- Every case to be reviewed once PCA/NCA discontinued, specifically looking for safety, incidents and learning points
- Reviews will consider input from Acute Pain Service (APRS) nurses, APRS clinical lead, supervising medical team, and ward nursing team members.
- Each patient will have a named consultant in charge of their Pain Management
- After a period of 6 months we intend to present finding to the Clinical Governance Forum, with the intention of extending use of Fentanyl PCA/NCA to the wider paediatric patient group within RHC.
- We anticipate to have cared for around 10 patients in this time period. This appropriate first evaluation point will be under continuous review.
