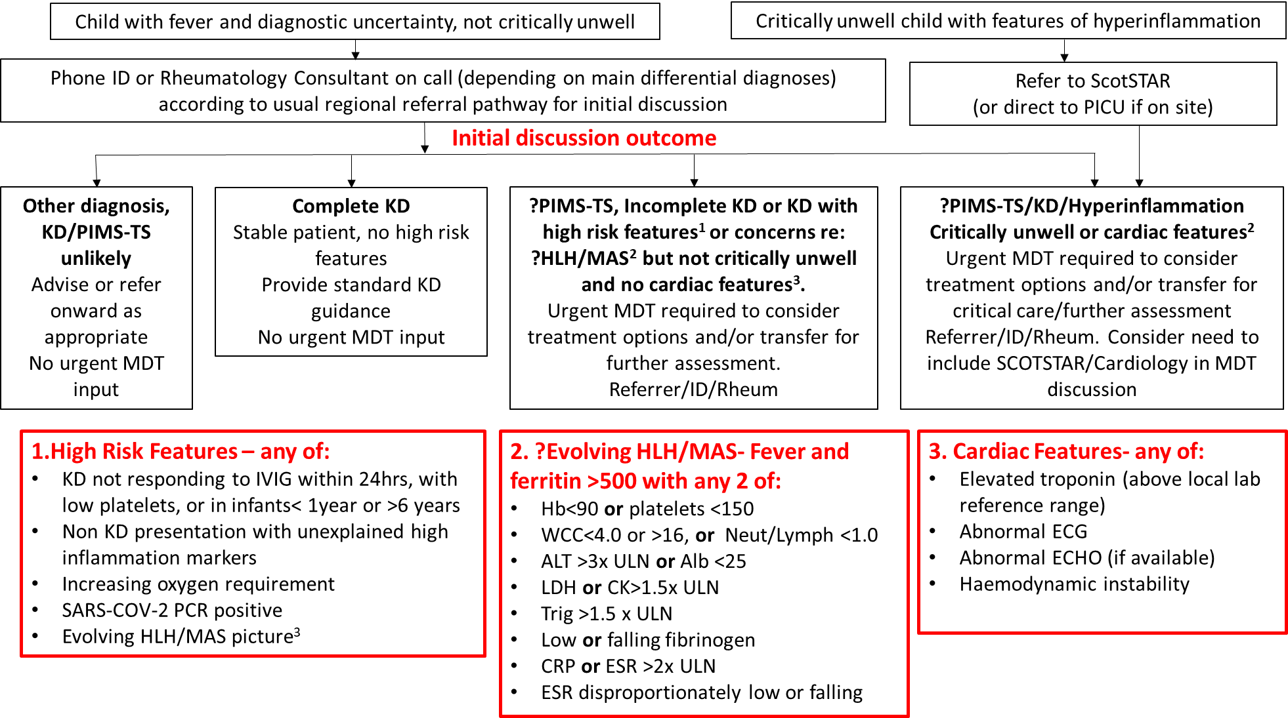
1.1 Kawasaki Disease (KD) Criteria
“Complete” KD is defined according to American Heart Association[2] criteria as :
Fever of at least five days in addition to 4 of 5 additional criteria:
- Conjunctivitis: bilateral, bulbar, conjunctival injection without exudate
- Lymphadenopathy: cervical, often >1.5cm, usually unilateral
- Rash: maculopapular, diffuse erythroderma or erythema multiforme
- Changes of lips or oral mucosa: red cracked lips, “strawberry” tongue, or diffuse erythema of oropharynx
- Changes of extremities: erythema, and oedema of palms and soles in acute phase; and periungual desquamation in subacute phase.
OR less than 5 days of fever but otherwise meeting all five AHA criteria.
OR less than 5 days of fever with coronary artery aneurysm or coronary dilatation.
1.2 Incomplete KD Criteria
Children (>1 year old) with fever for ≥5 days AND at least 2 other compatible clinical criteria listed above; OR infants ≤ 1year with fever ≥7 days without other explanation AND (for both age groups) CRP≥30 mg/L or ESR ≥40 mm/h AND
- EITHER the presence of any 3 or more of: anaemia for age (haemoglobin less than the lower limit of normal laboratory reference range for age); platelet count ≥ 450 x109/L or <140 x109/L; albumin < 30g/L; elevated ALT, WCC <15x 109/L; urine ≥10 WBC per high power field
- OR abnormal echocardiogram compatible with KD but without established CAA, with ≥3 of the following suggestive features: decreased left ventricular function, mitral regurgitation, pericardial effusion, or dilated but non-aneurysmal coronary arteries
1.3 Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS)
RCPCH case definition:
- A child presenting with persistent fever, inflammation (neutrophilia, elevated CRP and lymphopenia) and evidence of single or multi-organ dysfunction (shock, cardiac, respiratory, renal gastrointestinal or neurological disorder) with additional features (see below). This may include children fulfilling full or partial criteria for Kawasaki disease.
- Exclusion of any other microbial cause, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, infections associated with myocarditis such as enterovirus (waiting for results of these investigations should not delay seeking expert advice).
- SARS-Cov-2 PCR testing may be positive or negative.
Additional Features in PIMS-TS
Clinical Features:
- ALL: Persistent fever >38.5°C
- MOST: Oxygen requirement or hypotension
- SOME: abdominal pain, confusion, conjunctivitis, cough, diarrhoea, headache, lymphadenopathy, mucus membrane changes, neck swelling, rash, resp symptoms, sore throat, swollen hands and feet, syncope, vomiting
Laboratory
- ALL: abnormal fibrinogen, absence of potential causative organisms (other than SARS-CoV-2), high CRP, high D-Dimers, high ferritin, hypoalbuminaemia, lymphopenia
- Neutrophilia in most– normal neutrophils in some
- SOME: acute kidney injury, anaemia, coagulopathy, proteinuria, raised CK, raised LDH, raised triglycerides, raised troponin, thrombocytopenia, transaminitis
Imaging and ECG
- Echo and ECG – myocarditis, valvulitis, pericardial effusion, coronary artery dilatation
- CXR – patchy symmetrical infiltrates, pleural effusion
- Abdo USS – colitis, ileitis, lymphadenopathy, ascites, hepatosplenomegaly
- CT chest – as for CXR – may demonstrate coronary artery abnormalities if with contrast
Note: WHO refer to PIMS-TS as Multisystem Inflammatory Syndrome in Children (MIS-C). The WHO case definition is similar, but requires at least 3 days of fever and either evidence of COVID-19 on PCR or serology or a likely contact with COVID-19.
Case definitions are preliminary and subject to change. Check for updates here: