The recommended starting dose of Cenobamate is 12.5mg given once per day, typically in the evening. The dose should be titrated slowly to the recommended target dose of 200 mg per day.
Some patients, who do not reach optimal seizure control, may benefit from optimising the doses above 200 mg (increased by increments of 50 mg/day every two weeks) up to a maximum of 400 mg daily however this dose. The maximum upper limit for individuals <50kg is 4.5mg/kg/d.4
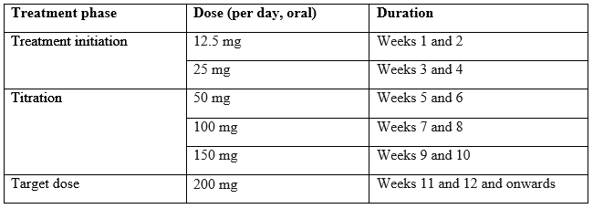
The recommended titration schedule is provided in table below.
![]()
Missed doses
If patients miss one dose, it is recommended that they take a single dose as soon as they remember, unless it is less than 12 hours until their next regularly scheduled dose.
Discontinuation
It is recommended that discontinuation be undertaken gradually to minimize the potential for rebound seizures (i.e., over at least 2 weeks) unless safety concerns require abrupt withdrawal.
Renal impairment
Should not be used in children with end-stage renal disease or patients undergoing haemodialysis.
Should be used with caution and reduction in the target dose in children with mild, moderate, or severe renal impairment. The maximum Cenobamate dose in the above scenarios is 300mg per day.
Hepatic impairment
Exposure to Cenobamate was increased in patients with chronic hepatic disease. No change in the starting dose is required but a decrease in target doses up to 50% may need to be considered. The maximum recommended dose in patients with mild to moderate hepatic impairment is 200 mg per day. It should not be used in patients with severe hepatic impairment.


