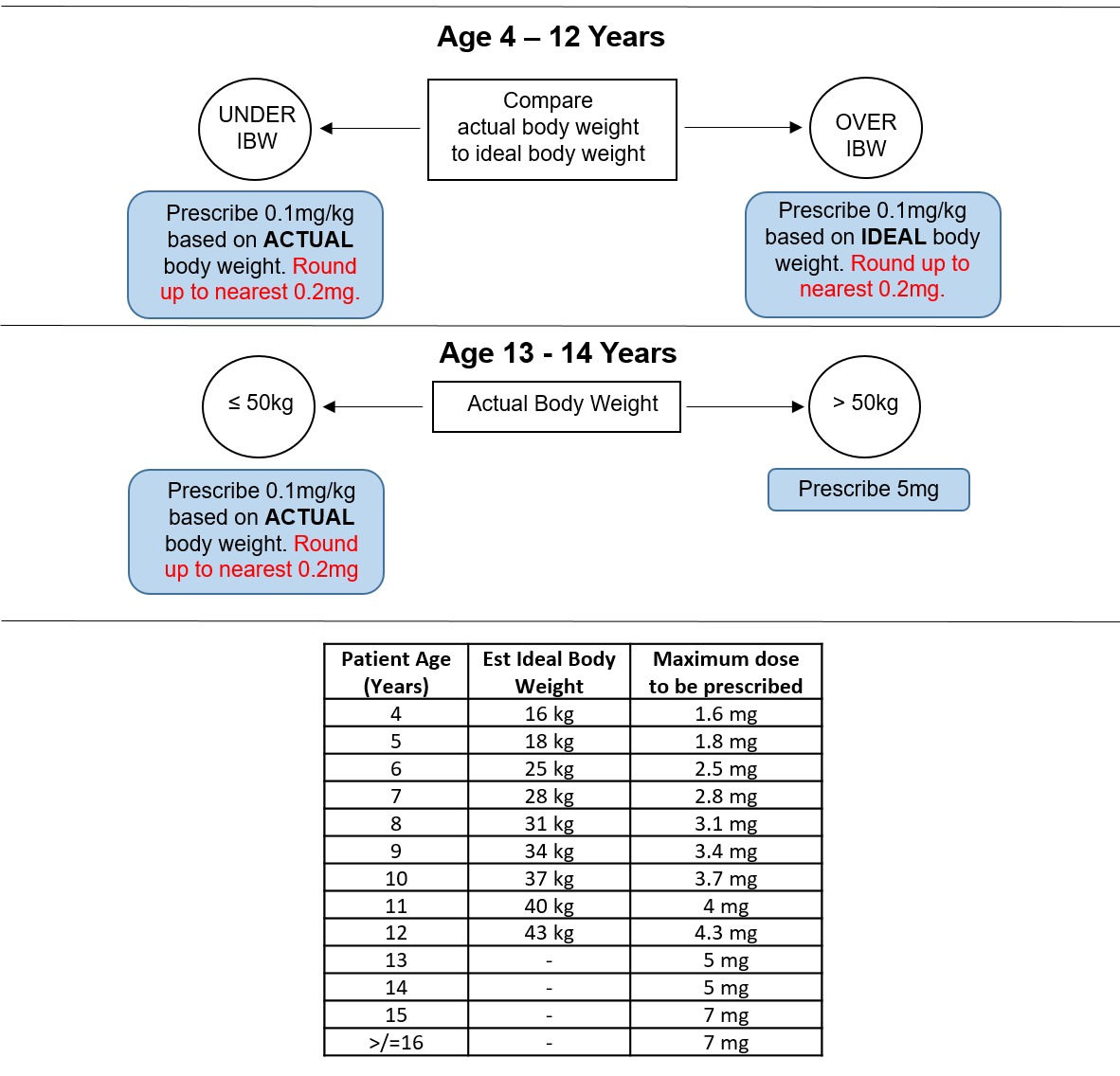
Patients should be prescribed the appropriate dose 4 times a day, with seven days supply for discharge. Ideal body weight (IBW) calculations have been calculated from APLS guidelines for patients between 4 and 12 years old (see table below). Queries should be directed first to the anaesthetist or surgeon managing the patient (contact in the appropriate theatre). If unavailable, duty anaesthetist x84842 or on-call ENT x84897 should be contacted.
Tonsil surgery at RHC Glasgow

Objectives
Guideline for children undergoing tonsil surgery with or without any other minor procedure at the Royal Hospital for Children, Glasgow.
The purpose of this guideline is to provide clarity and standardisation to the pathway of care for all children undergoing tonsil surgery with or without any other minor procedure at Royal Hospital for Children, Glasgow based on current evidence based research.
Tonsil surgery is one of the most common paediatric surgeries undertaken with around 1000 children having this procedure performed annually at the Royal Hospital for Children Glasgow [1]. The indications for this surgery are primarily for recurrent infections, sleep related disordered breathing (SRDB)/obstructive sleep apnoea syndrome (OSAS) or a combination of these symptoms [2]. In appropriately selected children tonsil surgeries performed on a same day discharge (SDD) basis have been proven to be a safe and effective way of managing post-operative care for these patients [3, 4]. At present, in our hospital, the discharge of these children following tonsil surgeries is undertaken on an ad hoc/case by case basis. The aims of this protocol are to provide clarity and standardisation to the pathway of care for all children undergoing tonsil surgery. It must be noted that even with optimal cares that some children who have their surgeries planned as SDD will ultimately require an overnight stay.
Definitions:
Tonsil surgery: This includes all tonsil surgeries including both tonsillectomy and tonsillotomy.
Other minor procedures: Any other procedure or procedures that would routinely undertaken on a same day discharge basis. ENT procedures that would be categorised in group include adenoidectomy, examination of ears and/or nose, removal of foreign body ear and/or nose, nasal cautery, grommet insertion or removal, division tongue tie and submucosal diathermy to inferior turbinates. Non-ENT procedures in this group will decided on a case by case discussion with the operating surgeon/s and anaesthetist prior to surgery.
Patient identification in Outpatients
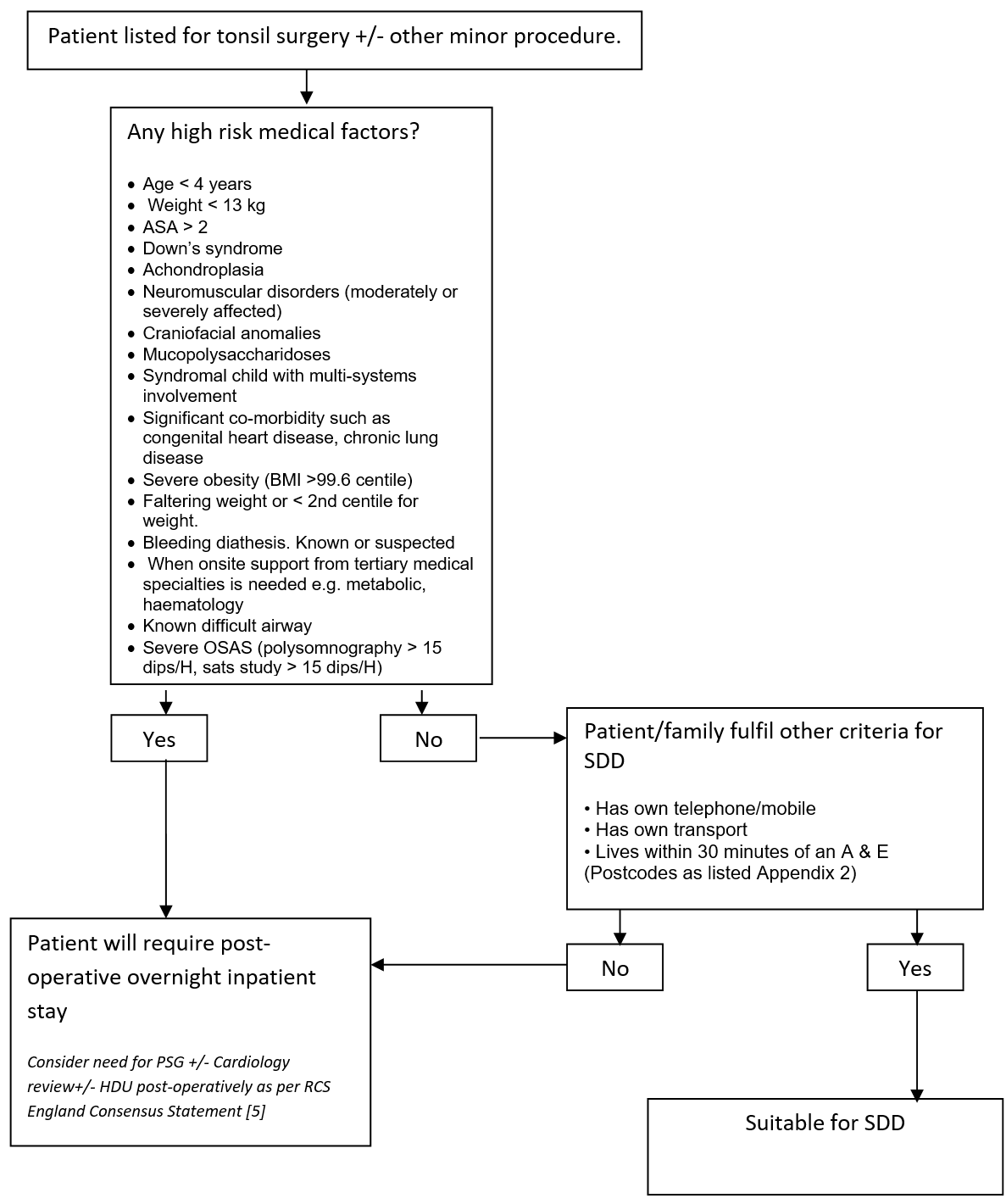
Patients listed for tonsil surgery with or without any other minor procedure at time of initial consultation will be assessed by the listing surgeon or nurse specialist as to appropriateness on a medical basis for SDD. Absolute exclusion criteria are listed in Appendix 1. Surgeons will discuss the possibility of SDD with patient and family at the time of consultation. If agreed then the listing clinician will either in the “Remarks” box “suitable for SDD” or “SDD” or in the length of stay mark this as “0”. Sleep studies (overnight saturation monitoring or polysomnography) for children listed for this surgery will be undertaken as per the recommendations in the “Tonsillectomy and adenoidectomy in children with sleep-related breathing disorders: consensus statement of a UK multidisciplinary working party” [5] and are otherwise not routinely required, unless at the discretion of the listing surgeon, or as per the recommendation of the “Safe Delivery of Paediatric ENT Surgery In The UK: A national Strategy. A combined Working Party of the British Association for paediatric Otolaryngology (BAPO), ENT UK, The Royal College of Anaesthetists (RCoA) and the Association of the Paediatric Anaesthetists of Great Britain and Ireland” [4].
Pre-assessment Clinic
The Pre-assessment Nurses will assess the patient within two weeks of surgery. They will check/confirm that the listing clinician has listed the child for SDD. They will confirm that no significant changes have occurred in the child’s general medical status. They will re-assess for appropriateness of SDD including medical and social factors as described in this document.
Social factor questions are listed in Appendix 1. Postcode look up in Appendix 2.
Where a child who was previously identified as being a potential candidate for SDD is felt to be ineligible they will inform the family and contact the ENT Secretaries to alert them to this.
Where appropriate the Pre-assessment Nurses will counsel children and their families regarding the SDD protocol.
The Pre-assessment Nurses will also counsel families to obtain their first line post-operative analgesia (paracetamol and ibuprofen) from a Pharmacy First Pharmacy. They will also counsel that their child's surgery may be undertaken on a same day discharge basis.
When seeing children face to face a weight will be obtained and uploaded to iGrow.
The Pre-assessment team will email an SBAR to DSU ward the night before surgery. The SBAR will include which tonsil patients are appropriate to have their surgery undertaken as SDD surgery patients.
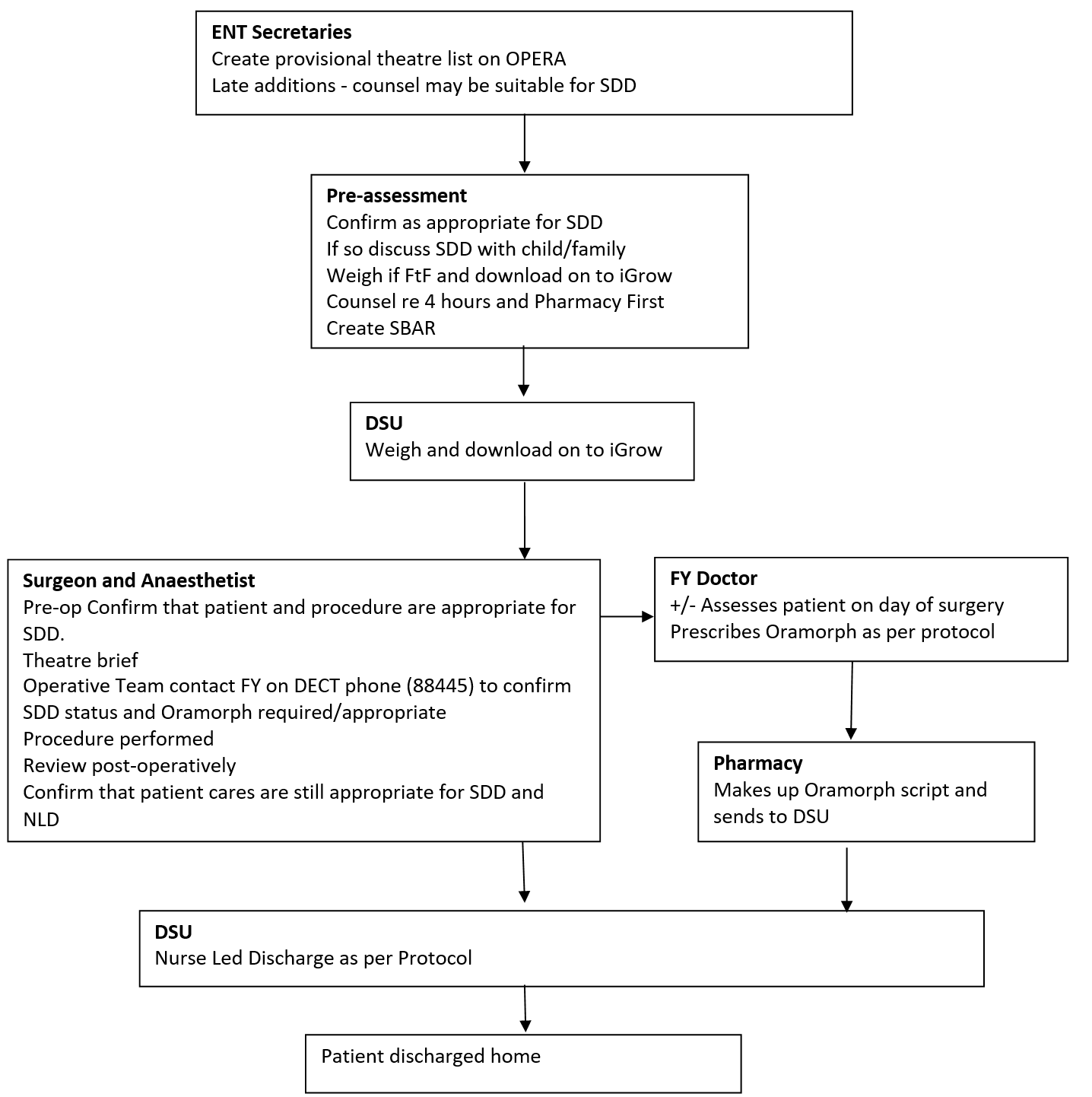
ENT Secretaries
At present up to 20% of children will not be able to be pre-assessed (personal communication), due to short notice changes and other variables. If the listing clinician has identified a patient, who it was felt could have their surgery performed as a SDD procedure and who has not attended/been able to be assessed by the Pre-assessment Team, the Secretary will contact the family to check availability and to let them know their date of surgery. The Secretary will ask the social factors as stated in Appendix 1 to assess appropriateness for SDD, and inform the family regarding the possibility of SDD. The Secretaries will then construct the Theatre list to accommodate this using this and the information obtained from the Pre-assessment Team.
Postcode criteria will be used to identify patients suitable for SDD. These postcodes must be within a 30 minute drive of an A & E in the Greater Glasgow and Clyde and Lanarkshire NHS Trusts. (included are all G postcodes except G63, G82-4, PA postcodes PA 1-19, ML postcodes ML1-11).
The patient will be re-assessed from a medical status both pre-operatively and post-operatively by the Anaesthetist and the Surgeon on the day of surgery and a final decision will be made as to whether the patient may potentially go home the same day.
The operative team will discuss patient suitability for SDD at the Theatre Brief. Following the brief the DSU FY will be contacted on their DECT phone by the operative team to confirm which patients are deemed appropriate for SDD.
This will then be communicated to the DSU Nursing Staff. The patient’s operation note must include “suitable for Nurse Led Discharge at 4 hours post-op”. Post-operative cares will be undertaken in the Day Surgery Unit (DSU) Ward.
Foundation Doctors
The FY attached to the DSU will write a prescription on HEPMA/IDL for Oramorph using the weight from iGrow/notes at the time of review in the DSU. Morphine will be prescribed per protocol (Dr N Willis).
Pharmacy
The Pharmacy will make up Oramorph prescriptions as described above. These medications will be dispensed from the second floor Pharmacy. Along with this, pre-marked syringes using a proven technique will also be dispensed.
Ongoing Analgesia requirements
Patient’s families will be advised to contact the ENT Department directly if following discharge at one weeks time that they feel their child requires a repeat prescription for oral morphine. A log of these requests/telephone calls will be kept. If morphine, as analgesia, is required beyond a send week the child will be reviewed in A & E by the ENT Team.
Please see Appendix 4
Discharge
Discharge from the DSU will be nurse led, as per the Nurse Led ENT Surgery Protocol (Appendix 3). Patients who have been identified as potential SDD patients will be assessed initially at 4 hours post-operatively, as per protocol, to see if the meet the discharge criteria, then 1- 2 hourly afterwards until decision to stay overnight or discharge is made. DSU Nursing Staff will counsel children and their families regarding risks, when to seek medical attention and the usage of post-operative analgesia.
Post-operative Patient information
All patients will be discharged with an IDL and a Tonsil Surgery post-operative information sheet. The latter will include advice regarding an effective Oramorph regime and the side effect profile to be aware of, as well advice regards post-operative bleeding. Paracetamol and Ibuprofen will be dispensed as TTO.
Post-operative discharge including analgesia
There is no proven post-operative analgesia formula that is most effective. Ibuprofen and paracetamol are both effective and appear to be more effective when given concurrently [6, 7]. There is good evidence that in spite of regular use of paracetamol and ibuprofen that a large percentage, around 75% of children post tonsillectomy, are still in significant pain and parents would and do use additional pain killers if available [8 -12]. Internationally there is significant variation in which opioid is recommended post-operatively and whether this should be prescribed regularly or as required [13, 14]. There is some evidence demonstrating effective analgesia and high parental satisfaction ratings [15] as well as decreased contact with out of hours/GP if children are sent home post-operatively with regular Oramorph as post-operative analgesia [10]. Children will therefore be sent home with a prescription for 100 micrograms/kg 6 hourly for 7 days, as is done in other Paediatric Children’s Hospitals in the United Kingdom (personal communication Alder Hey Children’s Hospital).
Post-operative stay
There is good evidence to support the safety of decreasing routine post-operative observation period with some authors advocating that this should be as short as 3 hours [16-18]. The patients who have been identified as suitable for SDD and who fulfil the criteria for Nurse Led ENT Discharge Protocol will now be discharged at 4 hours post-operatively.
Post-operative advice regards ongoing requirement for analgesia
For children with ongoing requirements for simple analgesia, families will be advised at Pre-assessment and again at discharge as well as being given a Discharge Information Sheet recommending that they are to attend Community Pharmacy for further prescription/dispensing. For children with ongoing requirements for ongoing requirements for opioid analgesia, families will be advised at discharge and will have a Discharge Information Sheet recommending that they return to the Hospital Pharmacy for a repeat dispensing. This information will be conveyed to the family at the Pre-assessment Clinic and again post-operatively by the discharging nurse as well as in the form of a patient information sheet.
Audit of Process / QI
All patients discharged to ward care still on SDD pathway who subsequently are not discharged on the day of surgery must have a “failed sdd pathway” form (Appendix 3) completed and handed to either Mr Clement or Dr Willis.
Social
- No access to a telephone/mobile phone
- No access to own transport
Geographical
- Living more than 30 minutes drive from a hospital with an A & E
- (Postcode based – please see Appendix 2)
Medical
- Age < 4 years
- Weight <13 kg
- ASA > 2
- Down’s syndrome
- Achondroplasia
- Neuromuscular disorders (moderately or severely affected)
- Craniofacial anomalies
- Mucopolysaccharidoses
- Syndromal child with multi-systems involvement
- Significant co-morbidity such as congenital heart disease, chronic lung disease
- Severe obesity (BMI >99.6 centile)
- Faltering weight or < 2nd centile for weight
- Bleeding diathesis. Known or suspected
- When onsite support from tertiary medical specialties is needed e.g. metabolic, haematology
The following are postcodes suitable for children to have their tonsil surgery undertaken on a SDD basis.
- All G postcodes except G63, G82-4
- PA postcodes PA 1-19 inclusive
- ML postcodes ML1-11 inclusive
Other postcodes will be considered if within half an hour drive of an A & E.
Please see Separate document ENT Nurse Led Discharge
Sedative Pre-Medication
The use of sedative pre-medication is not an absolute contra-indication for SDD tonsil surgery, though it may make it less likely for the patient to meet the discharge criteria within 4 hours post-operatively. Each case will be judged individually, and patients not meeting the discharge criteria at 4 hours must be discussed with the responsible surgical team and anaesthetic team before a decision is made to continue with SDD. If a patient is not meeting the discharge criteria by 6 hours post-operatively then an inpatient overnight should be considered.
Intra-operative fluids/Fasting
The following should be considered in appropriate cases
- Use of intra-operative fluids should be considered as these will reduce the risk of PONV associated with anaesthesia / opioids.
- Reducing the period of fasting pre-op, keeping fluids going until as close to surgery as we are comfortable. Hospital policy is clear fluids up until 1 hour pre-operatively. Consider utilising sip-until-you-send.
Steroids
The use of dexamethasone should be considered as there is reasonable evidence to support its use to reduce PONV and pain in adult patients undergoing tonsillectomy.
Analgesia / Anti-emetics
Tonsil surgery will, with very few exceptions, require intra-operative opioid analgesia for satisfactory post-op pain scores and return to oral diet. Intravenous paracetamol and diclofenac will have a synergistic analgesic effect with parenteral opioids, as well as reduce overall opioid requirement. Unless contra-indicated, one or both of these analgesics should also be considered intra-operatively.
With the high likelihood of intra-operative opioid use and the risk of blood in the stomach, patients undergoing tonsil surgery can be considered to be relatively high risk for post-operative nausea and vomiting (PONV). Prophylaxis using 2 different anti-emetics intra-operatively is recommended. This will encourage early oral diet post-operatively.
For post-operative pain relief, regularly prescribed analgesia is more reliably administered than if prescribed on a PRN basis. For this reason, regular oral paracetamol (15mg/kg) and regular oral ibuprofen (5-10mg/kg) should be prescribed on the drug administration chart. Care must be taken to document intra-operative doses and to ensure timings of regular doses do not breach the minimum times recommended between doses. For breakthrough analgesia, oral morphine should be prescribed (0.1 – 0.3mg/kg) 4 hourly as required. Care should be taken not to give too high a dose to higher risk patients with severe symptoms of obstructive sleep apnoea.