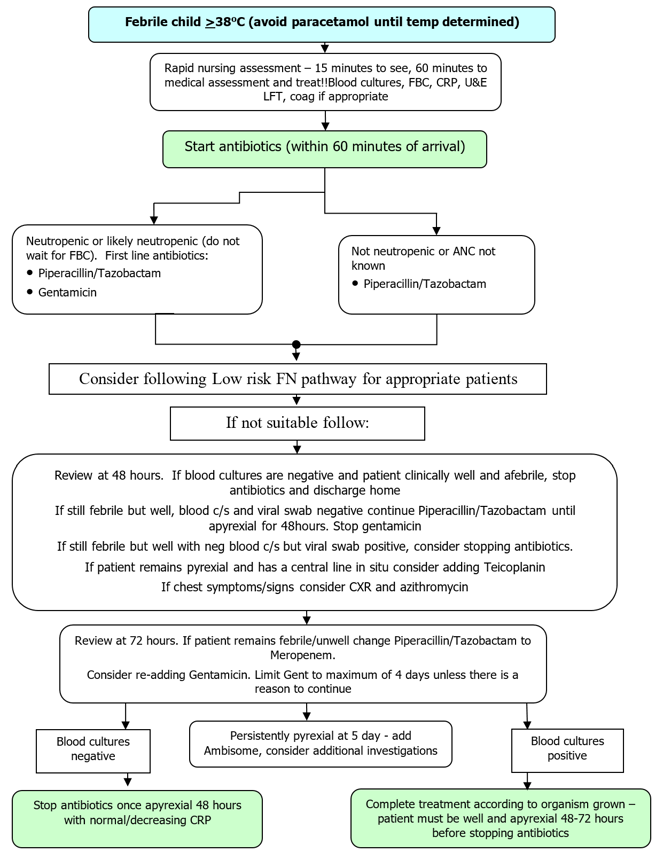
Neutropenia is defined as a neutrophil count of <1 x 109/L and patients who are neutropenic are vulnerable to overwhelming infection. The frequency and severity of infective episodes correlates with the degree and duration of neutropenia and is particularly marked in children whose neutrophil count is below 0.5 x 109/l. All children undergoing chemotherapy or targeted treatment for cancer and those who are within 3 months from the completion of treatment are at risk of severe infection. Most children present with a fever. Children can also present with low temperature, or just unwell, specifically those who are on steroids and those with Down’s syndrome.
Paracetamol should not be given until the decision to treat has been taken because it may mask a fever. Particular care should be exercised with HSCT patients who are neutropenic. It is always better to over rather than under treat these patients. These children can deteriorate rapidly.
If a patient deteriorates after using/flushing their central line, consideration should be given to siting a peripheral cannula and stopping using the line.
All Haematology/Oncology patients admitted overnight who are ill, must be seen by the most experienced middle grade doctor on for hospital cover who should discuss the patient causing concern with the consultant Haematologist/Oncologist on-call.
All Patients admitted with fever and at risk neutropenia are managed as per this policy. Some patients may be eligible for early discharge and outpatient management and are managed as Low Risk (LR) arm. Others are managed as per standard inpatient FN arm of this policy.
Aim of Low-risk FN management:
|
The Objectives of the Low risk febrile neutropenia (FN) management process is to:
|
|
This document is designed to provide a brief background and practical application of management of all patients who present with febrile neutropenia. This includes management of high-risk patients who require hospital admission and inpatient management and a tiered, shortened admission process and early discharge for Low risk FN. This process has been adapted from the Paediatric low-risk FN programme developed by Gabrielle Haesuler, National Centre for Infections in Cancer, Australia, and in collaboration with Jess Morgan and Bob Phillips, University of York, and the CCLG team including Barry Pizer, Sujith Samarasinghe, Richard Grundy, and Jessica Bate. |
Background to Risk Stratification:
Recognising that many children with FN remain well throughout admission, and that clinically significant infections are rare, much work has been done to attempt to identify patients with “low-risk” FN. This is important because management of FN episodes accounts for a considerable percentage of bed usage, which is expensive and inconvenient to patients and their families.
A number of strategies designed to reduce the duration of inpatient stay and/or early use of oral antibiotics have been evaluated as non-inferior to existing inpatient intravenous protocols. Seventeen paediatric FN clinical decision rules (CDRs) that risk stratify children with cancer and FN for infection have been derived.
Validation studies tend to show the rules differentiate between risk groups less well when applied to different datasets. The data from the prospective multisite (n=8) Australian-PICNICC study which enrolled 858 FN episodes in children with cancer were used to recalibrate the SPOG (Swiss) rule. This recalibration defined three equally weighted factors (WCC, platelets and chemo intensity). The AUS (Australia-UK-Swiss) rule was then validated in the ‘PICNICC+’ dataset, including over 1500 evaluable episodes of FN from the UK, Europe, North and South America. Published: https://doi.org/10.1002/pbc.28580. The relationship between AUS score and significant bacterial infection/bacteraemia is shown below:
Table 1. Australian data:
|
Score |
0 (n = 84) |
1 (n = 298) |
2 (n = 284) |
3 (n = 192) |
|
Bacteraemia |
3 (3.6) |
22 (7.4) |
36 (12.7) |
47 (24.5) |
|
Bacterial infection |
9 (10.7) |
49 (16.4) |
66 (23.2) |
74 (38.1) |
|
Mortality |
0 |
0 |
0 |
1 (0.05%) |
Table 2. PICNICC+ data:
|
Score |
0 |
1 |
2 |
3 |
|
No bloodstream infection (BSI) |
161 |
420 |
441 |
187 |
|
Bloodstream infection |
16 |
69 |
107 |
109 |
|
% BSI |
9.0% |
14.1% |
19.5% |
36.8% |
|
Death (any cause)* |
1 (0.6%) |
1 (0.2%) |
1 (0.2%) |
3 (1.2%) |
|
ICU |
|
|||
* Deaths in score 1 & 2 were related to disease. Death in score 0 was post-transplant patient with adenoviral reactivation. ICU admissions in this data set came mostly from in-patient episodes, and all were assessed at presentation as ‘seriously clinically unwell’. The 855 case prospective evaluation of FN episodes has demonstrated that by 24 hours, 80% of positive blood cultures will have ‘flagged’.
Graph 1. Time taken for flagging positive blood culture
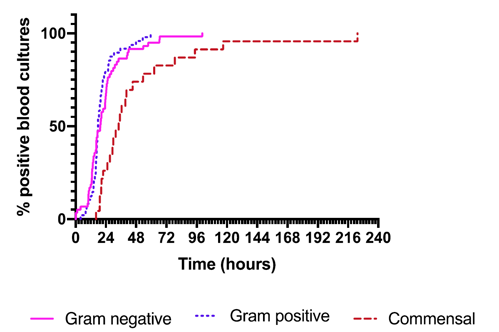
The CCLG Supportive Care Group is very encouraged by the data above with respect to the safety of the AUS Clinical Decision Rule and its associated management pathway.
The process has been piloted in Royal Children’s Hospital, Melbourne, where it led to a significant reduction in bed occupancy. In their pilot study 63 children out of 336 children with FN were able to safely receive antibiotics at home. The AUS-rule score, when combined with a safety assessment, can assist clinicians in determining when the patient can be safely discharged to home-based FN care.
Inclusion and Exclusion for Early Discharge:
Inclusion
- All children undergoing anticancer treatment with a risk of neutropenia
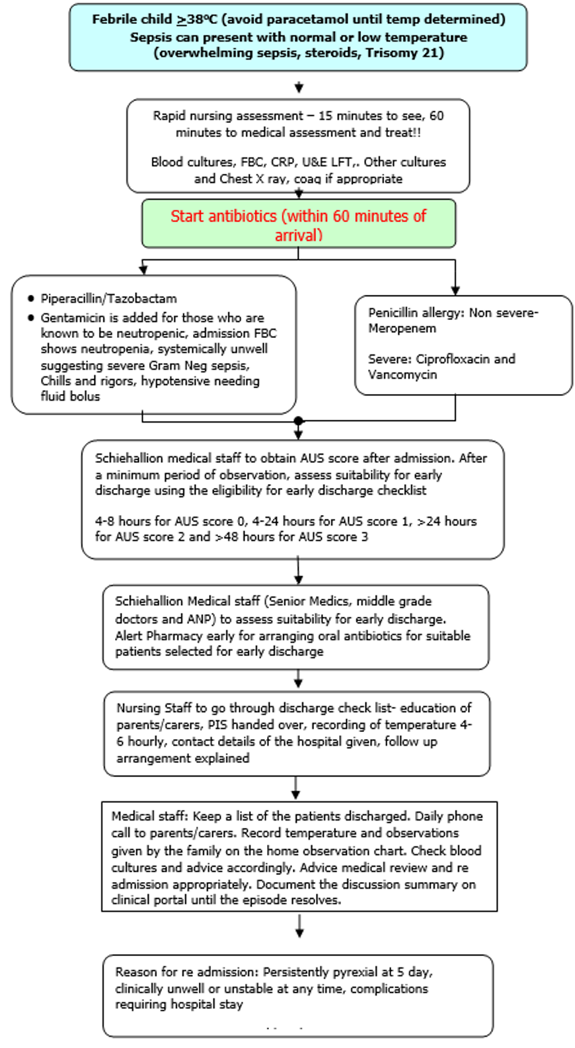
- Fever ≥ 38.0◦C Sepsis can present with normal or low temperature (overwhelming sepsis, steroids, Trisomy 21)
- Neutrophils: ANC < 1.0 x109 cells/L)
- Others: children on anticancer treatment with or without CVAD and unknown ANC at presentation, but at risk of neutropenia.
- Any child receiving chemotherapy who appears unwell but is not febrile or neutropenic may still need treating with antibiotics. If in any doubt as to whether antibiotics should be given it is usually preferable to err on the side of caution and give them. Discuss with more senior colleague if you are not sure.
Exclusion
- Children undergoing or post haematopoietic stem cell transplantation.
- Children with auto immune neutropenia (as there is no expectation of neutrophil recovery here)
- Benign haematology patients like sickle cell anaemia or aplastic anaemia