Viral Haemorrhagic Fever (VHF) patient pathway 2021 (Paediatric ED)
exp date isn't null, but text field is
See viral haemorrhagic fever risk assessment algorithm above.
Current Ebola outbreak areas can be found here
Inclusion criteria for high possibility of VHF:
Presence of fever >37.5 or positive history of fever in the last 24 hrs AND of travel to VHF endemic area in the last 21 days (see maps in appendix 1 below) AND:
Living in basic rural conditions where Lassa fever endemic (see maps in appendix 1 below)
OR
Visiting caves/mines, or having contact with/eating primates, antelopes or bats in a Marburg/Ebola endemic area (see maps in appendix 1 below)
OR
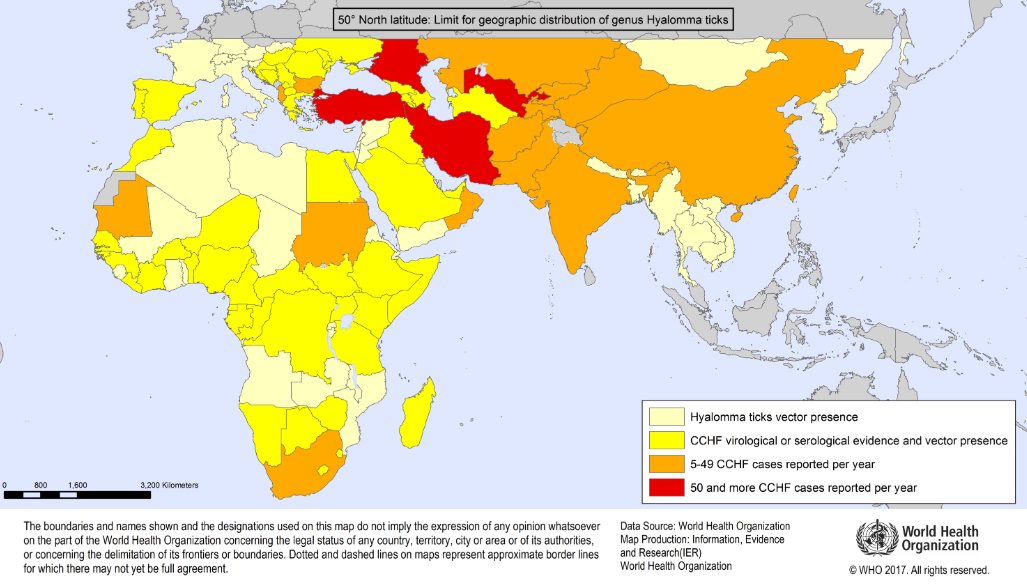
Travelling in an area where Crimean-Congo fever is endemic (see maps in appendix 1 below) AND sustaining a tick bite or crushing a tick with their bare hands OR had close involvement with animal slaughter
OR
The patient has active bleeding or extensive bruising
Or
Returning traveller from an area with current outbreak
If there is a high possibility of VHF, appropriate PPE should be donned (see PPE section below) and the patient moved safely to room 18 (negative pressure) in CDU
Investigation for malaria should proceed concurrently with VHF investigations
If a patient has previously had Ebola virus diagnosed (“Ebola survivor”) please discuss with the ID consultant on‐call. This document does not apply to them necessarily.
If these criteria are not met then there is a low possibility of VHF, malaria investigation should be prioritised and the patient managed in a single room with ensuite/commode.
If presenting with a child with a fever, ED reception staff should ask if they have visited one of the countries highlighted on the VHF and CCHF maps (see appendix 1 below) within the last 21 days.
Areas with endemic haemorrhagic fever include-
| Within Africa- | Mali Guinea Sierra Leone Liberia Ivory Coast Togo Benin Nigeria Gabon Congo DR Congo Angola South Africa Zimbabwe Kenya Uganda Sudan |
Out with Africa- | Afghanistan Albania Bulgaria Iran Kazakhstan Kosovo Kyrgyzstan Pakistan Saudi Arabia Serbia and Montenegro Tajikistan Turkey Uzbekistan United Arab Emirates West China West Russia |
- If there is a positive travel history to any of the above countries in that time frame AND history of fever, reception staff to ask patient to wait in the breast feeding room across from reception desk for the triage nurse.
- Reception staff to inform triage nurse and ED coordinator immediately.
- Reception staff to take a Trackcare screen shot identifying list of patients in the ED waiting room
- If a high risk of haemorrhagic fever is identified at triage nurse assessment then the decontamination of the breast feeding room should be discussed with infection control, and it must remain out of use until this is complete.
- If informed re: a child with a potential haemorrhagic fever by reception staff risk assessment should be undertaken in the breast feeding room wearing apron and gloves, and if risk of body fluid splashing onto face a surgical mask and eye protection. At this stage, and until the assessment has been completed, the patient would remain low risk.
- If patient is now deemed potentially high risk, then patient and family must be informed of this suspicion and informed that the patient is now in isolation, and that from this point staff wearing full Ebola PPE will attend to the patient’s needs.
- If patient identified in triage as high risk they should be instructed to remain in that triage room, to avoid contamination of multiple rooms, and informed that the next staff in attendance will be in full PPE.
- The patient should be transferred to room 18 in CDU once preparation is complete.
- If a patient identified as high risk has vomited or had diarrhoea or active bleeding (i.e. spills bodily fluids) in triage, the room doors should be closed, and the senior nurse in ED and infection control should be informed.
- Only staff in full PPE should re-enter contaminated room, to clean patient and bodily fluid spill, and transfer to Room 18 in CDU.
- If the patient spills bodily fluids in the waiting area, that area must be closed. Inform Public health and ID, take Trackcare screen shots as above, public health will deal with monitoring of contacts.
- Patients present in the waiting area should be moved through to ED/CDU cubicles as available. If this is not possible consider switching CDU waiting area/triage to red and move patients here. Green triage can move to clinic 3 or patients can be moved to the waiting spaces in minors. Until blood results confirm or refute VHF we are advised NOT to tell members of public in the waiting room that they have had possible exposure. They are not infectious at this point.
- New red patients arriving should be directed to CDU via the ambulance entrance if the waiting area is closed, and booked in and triaged in this area temporarily. Consider using clinic 3 or minors waiting area for new green patients.
- If a patient undergoing risk assessment has vomited in the waiting area, then the area should be cordoned off until the risk assessment is complete. If patient is high risk, the area should be closed and the area decontaminated as per Ebola IC Guidance (HPS). If a low risk patient vomits in waiting area, then area should be cordoned off and cleaned by cleaning up as per the body fluid spillage policy. See link 3 in references section for guidance.
- PPE should be donned by 2 nurses with assistance of buddy. Long hair should be tied up, consider using a surgical cap.
- Laminated instructions for donning are beside the trolleys containing PPE, located adjacent to the triage rooms. PPE is donned in room 1 in CDU.
- Radio walkie talkies should be sourced from the ED seminar room. Clocking in / clocking out board should also be collected from decontamination area for use by senior nurse to monitor staff in PPE.
- Those in PPE should be in communication with their buddy and the senior nurse on the ED floor.
- A large yellow waste bin should be placed inside room 18 (negative pressure room) in CDU from the area adjacent to the triage rooms. Yellow waste bags should be available inside the room. Vernigel sachets and bed pans / vomit bowls should be made available along with a commode (if required) which should stay in the room until patient has been transferred out. Actichlor Plus tablets, diluter bottle and Actichlor granules should be sourced to deal with body fluid spills. A disposable commode is presently on order.
- Monitoring equipment already in Room 18 should be used.
- If additional equipment/drugs needed, consider use of basin for clean individuals to place relevant equipment in then the buddy in their PPE to hand to person in full PPE.
Stored in ED major incident cupboard
Low risk
- Hand hygiene, gloves, plastic aprons, eye protection and fluid repellent surgical facemask as per current covid PPE requirements.
High risk
- Hand hygiene, double gloves, fluid repellent disposable gown/suit, apron, eye protection, FFP3 respirator, foot protection
Donning PPE
- See link to video guide at the top of this guideline
Removal (doffing) of PPE
- See link to video guide at the top of this guideline
- See poster in appendix 2 below
- For a planned removal of PPE the person in room 18 should inform the buddy and nurse via walkie talkie, with an already robed member of staff standing by. If heavily contaminated outer gloves, visor and apron should be removed in Room 18 before exiting to complete derobing in the antechamber with buddy assistance.
- Senior nurse should allocate the next 2 nurses to care for the patient if they are in the ED for >2 hrs.
Breech of PPE
- In the event of a breech in PPE within room 18, the buddy should be informed over the radio and the person in PPE should then exit the room and derobe with the assistance of the buddy.
- If a breech occurs in the derobing area, continue to derobe with assistance from buddy
- Infection Control should advise on immediate management of breech, and public health on follow up of individual who has breeched PPE.
- All potential and actual exposures should be reported to OH for follow-up. All staff looking after this patient become contacts whose names should be provided to OH.
- PPE should be donned by 2 nurses with assistance of a buddy. Long hair should be tied up, consider using a surgical cap.
- Laminated instructions for donning are beside the trolleys containing PPE, located in the ED major incident cupboard. PPE is donned in room 1 in CDU.
- Radio walkie talkies should be sourced from the ED training room. Clocking in / clocking out board should also be collected from decontamination area for use by senior nurse to monitor staff in PPE.
- Those in PPE should be in communication with their buddy and the senior nurse on the ED floor.
- A large yellow waste bin should be placed inside Room 18 in CDU from the area adjacent to the triage rooms. Yellow waste bags should be available inside the room. Vernigel sachets and bed pans / vomit bowls should be made available along with a commode (if required) which should stay in the room until patient has been transferred out. Actichlor Plus tablets, diluter bottle and Actichlor granules should be sourced to deal with body fluid spills.
- Monitoring equipment already in Room 18 should be used.
- If additional equipment/drugs needed, consider use of basin for clean individuals to place relevant equipment in then the buddy in their PPE to hand to person in full PPE.
- 0900-0000 ED consultant to be informed to decide best placed member of medical staff to don initial PPE and assess. This must be a senior decision maker.
- When no ED consultant on site this role will fall to ED registrar, but ED consultant must be informed and called to attend.
- Following medical assessment, or before it is complete, if requested via radio communication, the ID consultant on call should be informed of the attendance.
- Auscultation is not possible without breeching PPE. Work of breathing can be visualised and saturation assessed. Cap refill can be assessed, pulse unlikely through 2 gloves. BP will be available.
- Buddy should source a yellow bin for the de-robing area at the entrance to Room 18, a drape for derobing, and alcogel.
- Guidance from “Management of patients with possible VHF in GGC” on staffnet. See link at the top of this guideline
- Low risk of VHF:
- Samples from these patients can be handled in the laboratories as per routine samples.
- It is not necessary for the medical staff to inform the laboratory prior to sending samples.
- They should be investigated for other causes of fever as usual, e.g. malaria test, U&E, LFTs, Lactate, CRP, Glucose, FBC, Coagulation, Blood Cultures.
- No group and save samples should be sent.
- High risk of VHF:
- For paediatric VHF testing, collect 5ml EDTA (same as for FBC) & 5ml serum (yellow top).
- Grab Bags with the required blood bottles are in the trolley in the ED major incident cupboard.
- Prior to the results of the VHF test, local blood tests should only be performed if there is clinical need.
- Local blood tests may be deferred pending the VHF test results.
- No group and save samples should be sent.
- Do NOT take arterial blood gases.
- Blood sample packaging (high risk):
- See appendix 3 below for picture guide
- The blood bottles must be packaged in Category A transport boxes
- One container is required for each laboratory
- Each box contains a hard plastic container with lid, bubble wrap, PARAFILM “M” laboratory tape and absorbent material.
- Take only labelled blood bottles, parafilm and cannulation equipment into the room.
- After filling the bottles, the doctor should wipe his/her hands with an alcohol soaked wipe, then wipe each bottle individually. Seal the blood bottles with PARAFILM laboratory tape by wrapping the tape around the lid of each bottle like “cling‐film”
- An assistant, wearing gloves, should come to the door of the room, but not enter the room. The assistant should hold open specimen bags each containing absorbent tissue. The doctor from the patient’s room should drop a single blood bottle into each bag using a no touch technique.
- Away from the patient room, the assistant should seal each bag, then wrap each specimen + bag in bubble wrap before sealing it in the waterproof hard plastic container. The request form and sealed container is then put back in the cardboard box and sealed with the sticker provided prior to delivery to the lab.
- Packaged blood samples must be carried by hand to the haematology laboratory reception
- The ID Consultant should discuss with the UK Imported Fever Service (IFS) on 08 44 77 88 99 0 (Doctor on Call: 07789 031 672) prior to sending samples for testing.
- If samples are to be sent for VHF testing, the ID Consultant should call the SNVTS by contacting the On Call Consultant Virologist at the Royal Infirmary of Edinburgh through 0131 536 1000
- High risk children will likely be more safely nursed without their accompanying adult carers and this will be a stressful position for children and their families to understand. This must be approached and communicated sensitively to families.
- If parents are made aware of the risk of remaining in contact and are still insistent it may be possible for them to remain for a time in physical proximity in Room 18, with discussion with ID and infection control.
- Symptomatic siblings may have to be managed adjacent to the initial presenting child.
- Asymptomatic siblings should return home in the care of an appropriate adult and be monitored by public health.
- Asymptomatic adults will also be monitored at home by public health.
- Symptomatic adults must be transferred to QEUH. They should remain in Room 18 in CDU awaiting transfer.
|
ED Majors Consultant |
84059 |
|
ED Co-ordinator |
84585 |
|
Infection control Pamela Joannidis (Nurse Consultant and lead) Angela Johnson( Senior IPCN) Sharon Carlton ( Administrator) |
80600/80326 |
|
ID Consultant On Call: |
|
|
Microbiology lab |
89132 |
|
PICU Consultant |
84719 |
|
Virology lab (West of Scotland Virology Centre) |
50080 |
Geographic distribution of Crimean-Congo Haemorrhagic Fever
 Countries with confirmed cases of Lassa, Ebola and Marburg
Countries with confirmed cases of Lassa, Ebola and Marburg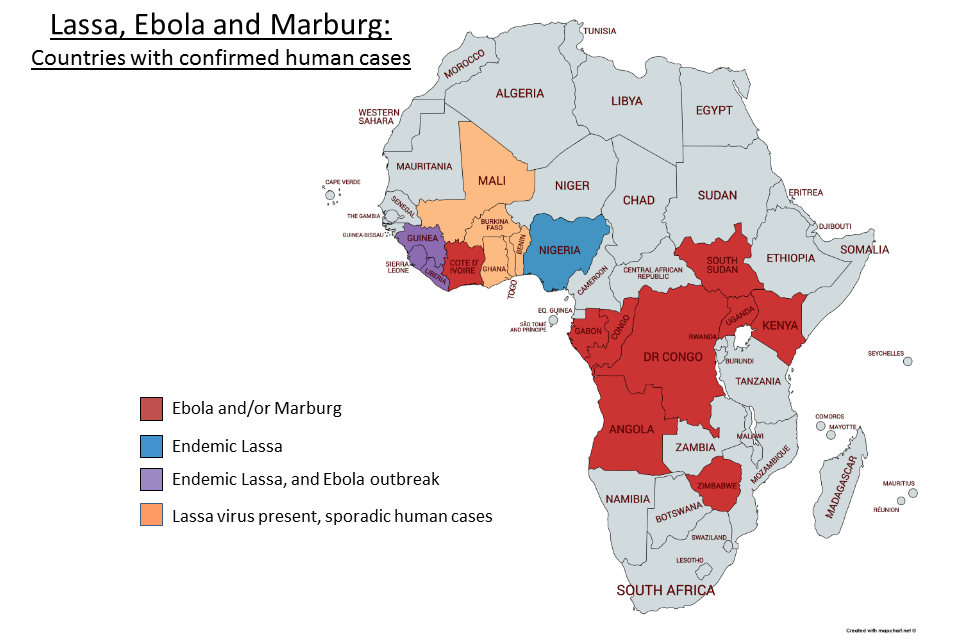
Grab Bags with the required blood bottles are in the trolley in the ED major incident cupboard.
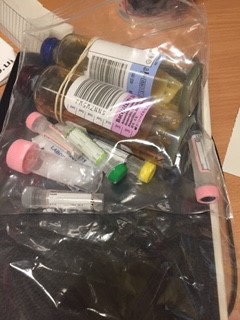
Take only labelled blood bottles, parafilm and cannulation equipment into the room.

After filling the bottles, the doctor should wipe their hands with an alcohol soaked wipe, then wipe each bottle individually. Seal the blood bottles with PARAFILM laboratory tape by wrapping the tape around the lid of each bottle like “cling‐film”
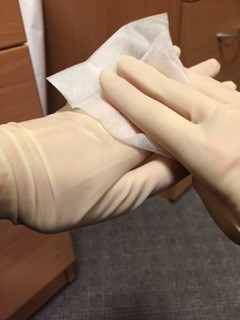
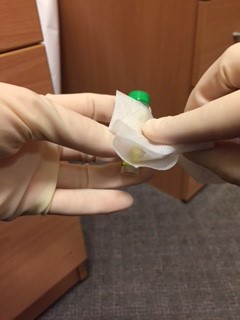
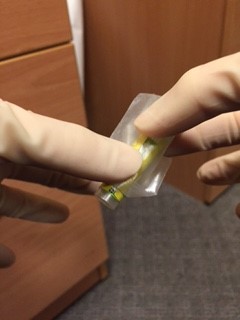
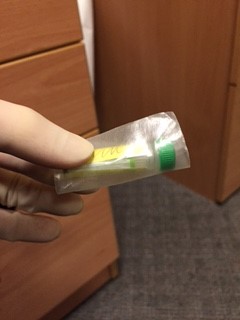
An assistant, wearing gloves, should come to the door of the room, but not enter the room. The assistant should hold open specimen bags each containing absorbent tissue. The doctor from the patient’s room should drop a single blood bottle into each bag using a no touch technique.

Away from the patient room, the assistant should seal each bag, then wrap each specimen + bag in bubble wrap before sealing it in the waterproof hard plastic container. The request form and sealed container is then put back in the cardboard box and sealed with the sticker provided prior to delivery to the lab.
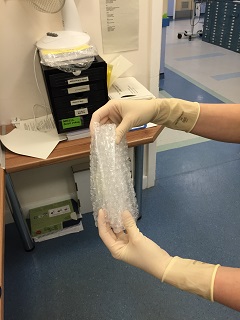





- Health Protection Scotland. Viral haemorrhagic fevers
- Public Health England / Department of Social Care. Viral haemorrhagic fever: ACDP algorithm and guidance on management of patients
- Health Protection Scotland. Viral Haemorrhagic Fever (VHF) Infection Prevention and Control Precautions Summary for the Hospital Setting (Version 3.1)
- NHSGGC. Possible Viral Haemorrhagic Fever (VHF) - Management in Adults and Children [Staffnet link]
Last reviewed: 11 June 2021
Next review: 30 June 2024
Author(s): Ciara Carrick
Approved By: Emergency Department

