Confirmed
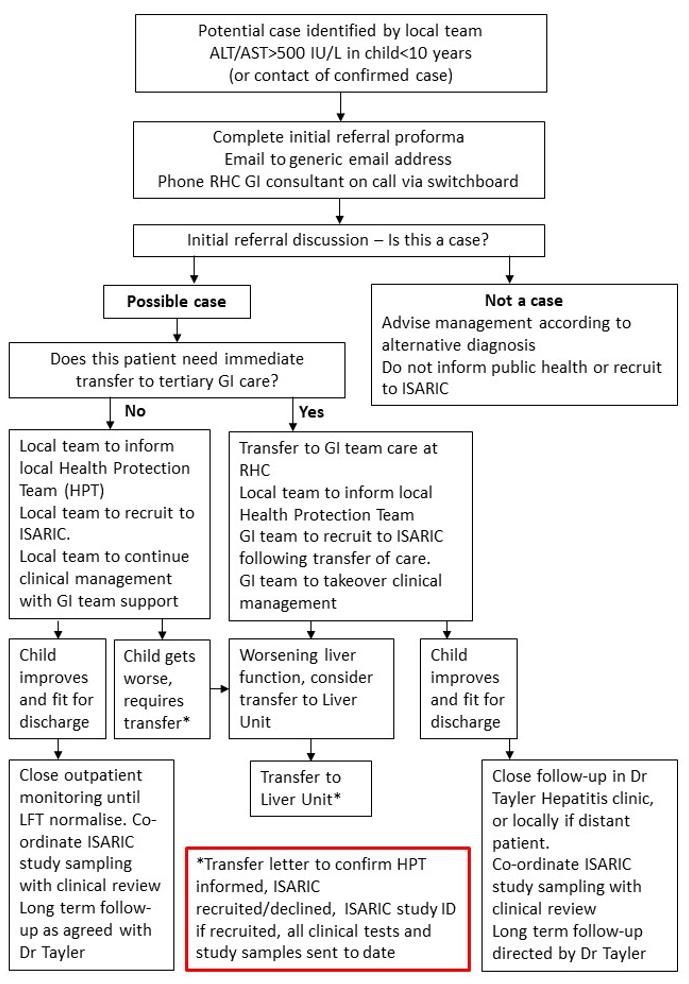
A person presenting with a serum transaminase greater than 500 iu/l (AST or ALT) without any known cause*, who is 10 years of age and under or a contact of any age of a possible or confirmed case, since 1 January 2022.
*If hepatitis A-E serology, CMV and EBV results are awaited, but other criteria are met, the case should be reported to PHS but will be classified as “pending classification”. Cases that meet the initial criteria but are later found to have alternative causes will be denotified.
This is a working case definition and subject to change. Note the PHS definition differs from the UKHSA definition. Please refer to the latest PHS updates to confirm. There should also be a low threshold for discussing with the GI team at RHC Glasgow any child up to 16 years who presents with transaminases >500 IU/L with no alternative diagnosis.
The ISARIC study case definition is wider and children up to 16 years with ALT or AST >400IU/L can be recruited to the study. Children who meet this definition, but not the PHS definition should not be reported as cases to public health.