Definition
External Ventricular Drains (EVDs) are a temporary system which allow drainage of cerebrospinal fluid (CSF) from the lateral ventricles of the brain.
EVDs are commonly used within neurosurgery for the management of patients who require drainage of CSF.
The EVD system is a closed system; breakage of the system would increase risk of contamination. Strict asepsis must be maintained.
Indications
Common indications include raised intracranial pressure (ICP) associated with:
- Head injury
- Subarachnoid haemorrhage
- Acute hydrocephalus
- Posterior fossa tumours
- Meningitis
Ventricular System Anatomy
There are four ventricles which comprise the ventricular system within the brain. The two lateral ventricles are the largest of the four, and are situated deep within the subcortical tissue, one each side of the midline. Each lateral ventricle communicates with the third ventricle through intra-ventricular foramina (Foramen of Monro). This third ventricle communicates with the fourth ventricle (located in the medulla) through the aqueduct of Sylvius (see Fig 1).
There are two lateral foramina and one median foramina located in the roof of the fourth ventricle which communicate with the subarachnoid space beneath the arachnoid membrane. The floor of the fourth ventricle is continuous with the central spinal canal.

Flow of CSF
The function of CSF is to provide buoyancy and support for the brain and spinal cord. It is a modified form of plasma consisting of water, glucose, protein, minerals and a few lymphocytes.
CSF is continuously secreted by the choroids plexus of the two lateral ventricles at a rate of approx. 20-25ml/hr (or 500ml/day).
At any one time, approx. 100-150ml of CSF are contained within the cerebral ventricles and the spinal cord. Once produced, it flows through the intraventricular foramen of Monro into the third ventricle and then through the single aqueduct of Sylvius into the fourth ventricle.
Once in the fourth ventricle, the CSF then flows into the subarachnoid space to flow around and over the brain, and into the spinal canal to flow around the spinal cord.
CSF is reabsorbed into the vascular circulation through the arachnoid villi at the saggital sinus. In health, the rate of reabsorbtion equals the rate of secretion.
An obstruction at any point in the flow of CSF will result in dilation of the cerebral ventricles and create a condition known as obstructive hydrocephalus. See Fig 2.
Failure of absorption of CSF at the saggital sinus will have the same effect on the ventricles but is known as communicating hydrocephalus.

Effects of CSF on Intracranial Pressure (ICP)
The Monro-Kellie hypothesis states that the skull is a rigid compartment filled to capacity with essentially incompressible substances (brain matter, blood and CSF). As such, an increase in one or more of the components will result in an increase in the overall pressure within the skull unless another component decreases in volume reciprocally.
ICP is thus affected directly by any changes in the volume of CSF within the brain. These changes in volume may be a result of –
- Change in the rate of secretion of CSF.
- Obstruction to the CSF flow within the ventricular system.
- Change in the rate of absorption of CSF.
Problems associated with the production, flow or absorption of CSF can cause a rise in ICP and therefore would be an indication for insertion of an EVD.
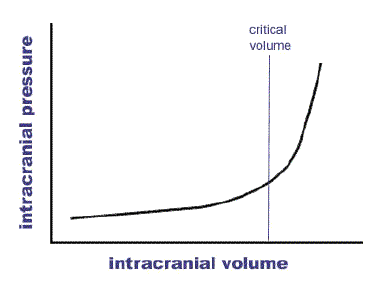
The above graph describes the relationship between pressure and volume within the skull. As can be seen, a reasonable increase in volume (irrespective of whether this is oedema, mass, blood or CSF) will be tolerated prior to ICP rising. The key is to intervene prior to reaching the critical point. Clinically this is associated with bradycardiac, hypertension and pupil changes. This is a pre-terminal event.








