


*Vascular access devices: care and maintenance


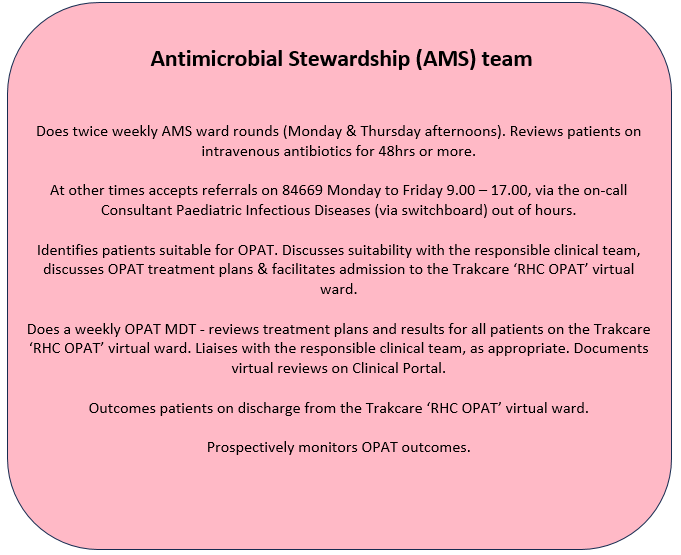
This pathway aims to facilitate and standardise OPAT provision for paediatric patients with complex infection treated with intravenous (IV) antibiotics. It should support the responsible clinical teams to manage their OPAT case load, improve communication with patients and their families, with primary and within secondary care, and facilitate standardised and consistent activity recording and clinical data capture. OPAT outcomes will be monitored and audited by the antimicrobial stewardship (AMS) team.
Outpatient parental antibiotic therapy (OPAT) is shown to be safe and effective for a wide range of infections in adults and children and is now a routine part of patient care in the UK. OPAT has many potential benefits. It allows earlier hospital discharge, moves patient care closer to home, and reduces the risk of hospital-acquired infections. Qualitative surveys have demonstrated increased patient satisfaction with paediatric OPAT in comparison with hospital-based care (1).
OPAT is a key component of the Scottish Government Interface Care Programme, which aims to safely provide an alternative to hospital admission or support early discharge.
The British Society of Antimicrobial Chemotherapy (BSAC) published updated good practice recommendations (GPR) for OPAT in adults and children in the UK in 2019 (2). These underpin the Scottish Antimicrobial Prescribing Group OPAT group key performance indicators (KPI), which aim to support optimum care for patients in Scotland accessing OPAT services. The GPRs and KPIs have informed development of this pathway.
The pathway aims to facilitate and standardise OPAT provision for paediatric patients with complex infection treated with intravenous (IV) antibiotics. It should support the responsible clinical teams to manage their OPAT case load, improve communication with patients and their families, with primary and within secondary care, and facilitate standardised and consistent activity recording and clinical data capture. OPAT outcomes will be monitored and audited by the antimicrobial stewardship (AMS) team.
Box 1. OPAT criteria
Clinical:
Social:
Carer:
Box 2. Intravenous access device
If patients are likely to require more than 7 days of intravenous antibiotics, please email Dr Phil Bolton & Dr Danni Seddon to discuss the most appropriate venous access device and placement.
Please see the GG&C guideline for advice on trouble shooting & removal.
Anything less than a 4Fr line should not be used for blood sampling.
Box 3. Treatment recommendations
Antibiotic: according to clinical indication & as discussed with AMS team, include a stop date.
Please also prescribe:
Monitoring:
Box 4. Safety netting:
Advise parents on safe care of the venous access device, to avoid getting it wet.
Advise parents to monitor for pain, redness or swelling at the venous access site.
If venous access is lost at home, but the child is otherwise well, advise to apply a plaster and to attend the ward as planned.
If concerns but the parent/carer does not think that the child needs urgent medical attention, advise to call the RHC switchboard (0141 2010000) and ask to speak to the on-call registrar for the responsible clinical team 9.00 – 21.00, either the hospital at night lead or the on call registrar for surgical specialties 21.00 – 9.00 (as detailed in the patient-held plan).
Advise to call 999/attend ED if the child is seriously unwell, has RED signs:
Ensure parent/carer has the OPAT patient information leaflet and a completed patient-held OPAT plan, has signed the parent/carer declaration.
Scan and upload a copy of the patient-held OPAT plan and parent/carer declaration to Clinical Portal.
Box 5. Information required on the IDL at time of hospital discharge/admission to OPAT