Respiratory management of preterm infants
exp date isn't null, but text field is
Objectives
This guideline updates the previous Respiratory Management of Preterm Infants guideline (June 2016). This reflects current evidence for the use of primary respiratory support with nasal CPAP in selected infants. It continues to provide guidance for the use of intubation and surfactant therapy or less invasive surfactant administration (LISA), for those infants who require escalation of support or who fail on CPAP alone. It also presents some of the current approaches to the use of postnatal steroids in preterm infants.
Audience
This Guideline is intended for use by the medical and nursing staff of the Neonatal Units in the West of Scotland. Please refer to Local Pharmacy guidelines for use of any medications mentioned.
Staff should be familiar with the function of all respiratory support devices (Resuscitaires, CPAP devices & Ventilators) in use within their local service.
Worldwide approximately 10% of deliveries are preterm deliveries and majority of this population need some form of respiratory support (CPAP, intubation, surfactant) at the time of delivery and thereafter. Every form of respiratory support is aimed at improving survival, survival without neuro-developmental impairment (NDI) and survival without chronic lung disease (CLD).
In spite of advances in neonatal medicine and improved survival, the incidence of CLD remains high. The incidence ranges from 30% among VLBW infants and 40% among infants <28 weeks1. CLD has a huge impact on health economics and it is a predictor of significant neuro-motor, developmental and behavioural sequelae 2,3.
This guideline presents strategies for initial respiratory support of the preterm infant at risk of respiratory distress syndrome.
- Primary therapy with nasal CPAP, with mechanical ventilation as a rescue strategy
- Primary therapy with nasal CPAP, with less invasive surfactant administration (LISA)
- Initial ventilation & surfactant administration, with consideration of early extubation to CPAP.
The choice of strategy will depend on a number of factors including:
- Size and gestation of the infant
- Exposure to antenatal steroids
- Condition at delivery
- Experience of the staff in the delivery unit
- Requirement for transportation to another hospital postnatally
Types of CPAP Machine & Interfaces:
The preferred method of CPAP delivery is by short binasal prongs or nasal mask using an Infant flow driver interface with humidification of the inspired gases (devices using this interface include – Infant Flow Driver, Viasys SiPAP and the Inspiration Systems Fabian ventilators / CPAP). This method of CPAP delivery, utilising the fluidic flip model, is more physiological, reduces the work of breathing and has been shown to be more effective. Where a device utilising such an interface is not available e.g. in labour ward, or for transfer to the neonatal unit, CPAP may be given via a face mask for transfer. The infant should be transferred to an appropriate CPAP device as soon as is possible.
CPAP initiation and Maintenance:
- CPAP should be started at pressures of 7-8 cm H2O and may be adjusted in increments of 1-2 cm H2O based on the clinical situation. However, if higher pressures are required this may indicate the need to consider surfactant and intubation.
- Each increment should be reviewed by as senior clinician.
- An initial oxygen requirement may be present during lung recruitment and this may be tolerated as long as this is stable or reducing and no other criteria for intubation are present.
- Caffeine citrate should be commenced as soon as possible for infants <30 weeks gestation (and for all <32 weeks gestation if there is poor respiratory drive). See Apnoea of Prematurity guideline
Complications associated with CPAP:
- Air leaks (pneumothorax, pneumo-pericardium and pneumo-peritoneum).
- Nasal excoriation, nasal septal injury (pressure necrosis), bleeding and secondary infection.
- Gastric distension and Feed intolerance. The former may be minimised by having an orogastric tube on free drainage to decompress the stomach. However, there is no evidence that gastric distension affects the time taken to reach full enteral feeds.
- Delay in surfactant treatment may lessen the surfactant effect.
Predictors of CPAP Failure:
Current evidence suggests that more than 50% of preterm infants do not manage on CPAP therapy alone. These babies will require escalation of respiratory support. Evidence from systematic review would suggest that the effects of surfactant are greater when given early in the disease process. It is therefore essential to identify early those infants who are going to fail CPAP alone. Such a decision is based on multiple factors and a thorough clinical assessment, which should consider the size and maturity of the infant, whether they received antenatal steroids, and the stage of their respiratory illness
(NB - lower thresholds should apply for an infant in the first few hours after birth).
The following factors should prompt a consideration of the need to administer surfactant +/- ventilate the infant.
- Worsening respiratory distress – As evidenced by increasing tachypnoea, recession etc
- Poor respiratory drive – As evidenced by periods of hypoventilation, despite caffeine therapy
- Recurrent apnoea - ≥6 apnoeas within last 6 hours requiring stimulation / increased oxygen or 1 episode requiring IPPV, despite caffeine therapy
- Persistent Increase in Fio2 requirement - Any infant with an FiO2 >40%, or in the first few hours, any persistent rise in FiO2 associated with an increased work of breathing
- Worsening respiratory acidosis - PCO2 8-8.5kPa with H+ >60 nmol/l.
- Haemodynamic compromise - Hypotension and poor perfusion requiring fluid resuscitation or inotropic support for > 2h.
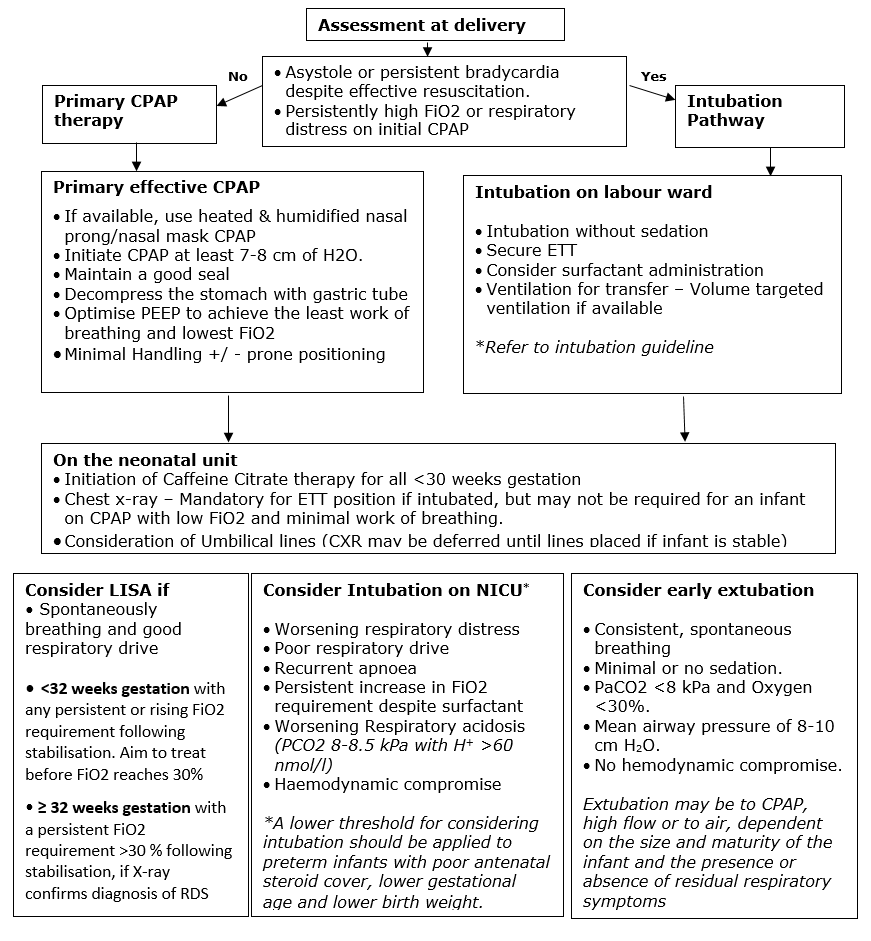
For infants with a good respiratory drive who are managed on primary CPAP and have increasing respiratory distress and a rising oxygen requirement due to RDS, less invasive techniques to deliver surfactant have been developed to reduce the need for mechanical ventilation. Less invasive surfactant administration (LISA) is the process by which surfactant is delivered directly into the lungs via a fine bore catheter inserted into the trachea while nasal CPAP is maintained.
Recent evidence suggests that a threshold FiO2 of 30% should be used for all infants <32 weeks gestation to reduce complications of untreated RDS. Where there are risk factors for RDS and a rising FiO2 trend or significant increased work of breathing, with evidence of RDS on chest x-ray, early surfactant administration via LISA prior to reaching current FiO2 thresholds may be considered at consultant discretion. While this technique is still relatively new, it is advisable that it is performed in the controlled environment of the NICU rather than in the delivery room. Use of a videolaryngoscope where available is also beneficial to ensure correct catheter placement prior to surfactant administration.
Indications for performing LISA:
- Perform LISAin infants <32 weeks gestation with any persistent or rising fiO2 requirement, following stabilisation on non-invasive respiratory support. The aim is to treat with surfactant before the fiO2 requirement reaches 30%, at any time in the first 72 hours. Or, as soon as possible if the FiO2 is already >30% following stabilisation.
- Perform LISAin infants >32 weeks gestation with a persistent or rising fiO2 requirement >30% following stabilisation on non-invasive respiratory support, if x-ray confirms the diagnosis of surfactant deficiency. Treatment may be considered at any time in the first 72 hours. At consultant discretion, LISA may be performed, without awaiting radiological diagnosis, in an infant with a higher initial oxygen requirement (fiO2 > 40%)
Contraindications to performing LISA:
Absolute Contraindications
- Imminent need for intubation as judged clinically by the attending senior clinician
- Maxillo-facial, tracheal or known pulmonary malformations
- Alternative cause for respiratory distress e.g. Congenital pneumonia
- No experienced personnel available to perform LISA
Relative Contraindications
- Severe RDS with high oxygen requirement, severe respiratory acidosis and widespread atelectasis on chest x-ray.
- Infants < 26 weeks gestation in a unit just starting to use LISA
- Pneumothorax requiring drainage
- Prominent apnoea, periodic or irregular breathing pattern, despite adequate caffeine citrate administration
Detailed procedure available in the West of Scotland LISA guideline
Intubation in the labour suite
Clinical assessment at the time of delivery should take into account the size and maturity of the infant, antenatal steroid therapy, and the condition immediately following delivery. The clinician may choose to intubate electively, on the basis of this assessment, or if one of the following is present.
- Asystole or persistent bradycardia despite effective face mask IPPV and lung inflation
- The infant demonstrates an increased work of breathing and consistently requires high FiO2 on initial CPAP
Where intubation is required in the delivery room this will normally be performed without sedation.
Refer to the intubation guideline to choose an appropriately sized ETT, determine the insertion length and methods of ensuring correct ET position.
Following correct ET placement, the clinician may choose to administer an initial dose of surfactant prior to transfer or they may elect to administer surfactant following a confirmatory CXR on arrival in NICU. Where it is decided to delay administration of surfactant until transfer to the neonatal unit there should be a delay of no more than 30 minutes. (MCQIC BPD reduction package)
Intubation in NICU
Intubation in NICU usually results from the infant having met the criteria for CPAP failure, although it may be required for other reasons, such as the need to transport an infant to another centre for ongoing care. A persistent and rising oxygen requirement in the first few hours after birth may be an indication for intubation, in particular if associated with an increased work of breathing. This may be especially relevant if there is evidence of worsening RDS despite surfactant administration via LISA. Later in the infant’s course, a higher level of FiO2, up to around 40%, may be tolerated if none of the other criteria are met
Unless the infant requires urgent intubation this should be performed with sedation and muscle relaxation.
Following intubation, a CXR should be performed promptly to ensure appropriate endotracheal tube positioning prior to surfactant administration.
In a ventilated infant, If FiO2 is rising rapidly consider DOPE
D - Displacement of ETT or CPAP prongs
O - Obstruction of ETT or nasal airway. Consider suction of ETT or nose as appropriate
P - Pneumothorax listen to air entry bilaterally, consider cold light / CXR
E - Equipment failure
If these are excluded, then this may represent worsening RDS and need for increased respiratory support. Senior review is advised
Detailed intubation procedure available in the West of Scotland Intubation guideline
Surfactant
Type - The surfactant currently used in all neonatal units in the West of Scotland is Poractant alpha (Curosurf).
Timing of administration – Early (<2h) surfactant therapy is more effective than delayed surfactant therapy. An initial dose may be given at birth in infants at high risk of RDS who are intubated in the delivery room, after an early (< 30 minutes) CXR if electively intubated in NICU or via LISA.
Initial Dose – The licensed initial dose is 100-200 mg / kg with evidence that the higher dose is more effective and results in the need for fewer repeat doses. Given the cost per vial, it is usual practice to give multiples of whole vials (120 / 240 mg vials available) to approximate this dose. If administering surfactant on labour ward where the weight is unknown it is reasonable to administer a whole 120mg vial and to consider a supplementary aliquot on arrival in NICU once the weight is known.
Repeat doses - Additional doses of surfactant may not be required for infants with low respiratory support requirements after the initial dose. One or two further doses may be administered if the infant continues to require high FiO2 or inspiratory pressures. The licensed dose for such additional treatments is 100 mg/kg, again usually giving a multiple of whole vials to approximate this dose.
Technique – In intubated infants, to ensure even distribution, ensure the correct length of ETT insertion. Administer via a fine bore catheter inserted down the ETT, ensuring that the catheter is slightly shorter than the length of the ETT. Administer as a single aliquot over 5-10 seconds (more rapid administration may induce a cough reflex forcing expulsion of part of the dose). Ensure there is no surfactant bubbling up into the ETT connector before reconnecting to a ventilator, as this may damage the sensitive flow monitor of the ventilator. (This may require a few manual breaths via a NeoPuff or Ambubag until surfactant is no longer visible in the ETT). For a few minutes only, keep the head in the midline and the ETT held above the baby to ensure the whole dose has been absorbed before returning the infant to a developmentally appropriate position for ongoing care.
Post surfactant management – Lung compliance may improve rapidly after surfactant administration. This will require close attention to weaning respiratory support, or the use of a volume targeted ventilation strategy in ventilated infants.
Caffeine therapy
All infants <30 weeks gestation and any infant <32 weeks with poor respiratory drive should be commenced on caffeine therapy routinely. This improves respiratory mechanics and is a prerequisite for the consideration of early extubation.
Ventilation strategies
Ventilation strategies should aim to minimize lung injury by avoiding overinflation.
Volume targeted ventilation (Volume guarantee (VG) / Targeted Tidal Volume (TTV)) as an adjunct to a patient triggered ventilator mode (Patient triggered ventilation (PTV) / Synchronised Intermittent Positive Pressure Ventilation (SIPPV) / Assist-Control (A/C)) has been shown to reduce lung injury and subsequent CLD. If such a mode is not used, then close attention will be needed to ventilation to ensure that appropriate weaning occurs as compliance improves.
pCO2 levels
It is unnecessary, and potentially injurious, to target “normal” CO2 values. CO2 values of up to 8-8.5 kPA may be tolerated, assuming the infant can maintain an acceptable H+ (<60 – 65 nmol/l).
Sedation/Paralysis
Routine use of sedation or paralysis is discouraged as it is known to prolong the time on ventilation. Infants should be regularly assessed for pain or discomfort and offered appropriate analgesia as indicated.
O2 saturation targets
Target of 91-95% O2 saturations
Consideration of early extubation
If an infant has a good response to surfactant then consideration should be given to early extubation to non-invasive respiratory support. A full clinical assessment is required, but if early extubation is considered the infant should fulfil the following criteria
- Consistent, spontaneous breathing
- Minimal or no sedation
- PaCo2 <8 and Fio2 <30%
- Mean airway pressure of 8-10
- No hemodynamic compromise
Extubation may be to CPAP, high flow or to air, dependent on the size and maturity of the infant and the presence or absence of residual respiratory symptoms.
This section presents the postnatal steroid therapies currently in clinical use. There is evidence for each treatment strategy, however research in this area is ongoing and as such recent consensus statements are included below. Practice may differ between neonatal units as agreed by the clinical team.
Early low-dose systemic hydrocortisone
Evidence is emerging that early low-dose prophylactic hydrocortisone reduces CLD rates and mechanical ventilation days. The rationale for use is that steroid replacement helps to achieve physiological levels that may be supressed due to reduced cortisol production in extremely preterm infants. Its use is increasing in the management of preterm infants, although is not yet widespread.
The PREMILOC trial is the largest multicentre RCT comparing early low-dose hydrocortisone with placebo in infants born <28 weeks gestation. The trial demonstrated increased survival without CLD at 36 weeks gestation in the hydrocortisone group, with the effect being more pronounced in those born at 26-27 weeks gestation. In addition, more infants in the hydrocortisone group were successfully extubated by day 10 of life. In the 24 - 25 weeks subgroup, hydrocortisone treatment was associated with an increase in the rate of late-onset sepsis. There was no associated increase in GI perforation risk. In the 24-25 weeks subgroup, improved neurodevelopmental outcomes were observed at 2-year follow-up.
The MCQIC BPD reduction bundle suggests - Low-dose hydrocortisone should be considered for all infants born < 28 weeks for the first 10 days of life
Eligible infants:
- Infants born at ≤ 28 weeks gestation
Dosing regime:
- Total 10-day course of IV hydrocortisone commenced on day 1 of life
- Day 1-7 dose: 0.5mg/kg/dose 12 hourly
- Day 8-10 dose: 0.5mg/kg/dose 24 hourly
Considerations:
- Avoid concomitant use of NSAIDs (Indomethacin or Ibuprofen)
- Due to the 10-day IV treatment course, consideration as to whether peripheral IV access should be sited to allow completion of course if central venous lines are no longer required for nutrition. In discussion with pharmacy, the course can be completed orally if obtaining further IV access is deemed inappropriate by the attending consultant.
Post-natal systemic dexamethasone
Treatment with systemic dexamethasone has been used to facilitate extubation, reduce mechanical ventilation days and reduce CLD. Associated adverse effects include hyperglycaemia, hypertension, growth failure and, when used in the first 7 days of life, increased risk of GI perforation and poorer long-term neurodevelopmental outcomes. Therefore, the adverse effects of early dexamethasone given in the first week of life outweigh the potential benefits.
Indications:
- Preterm infants at high risk of BPD
- Age >7 days
- Ongoing need for mechanical ventilation, with high O2 requirement and inability to wean support
- Infants who are ventilated on day 15 should have received steroids for prevention of BPD or documentation of consideration. (MCQIC BPD reduction package)
Course:
- A tapering course of low-dose dexamethasone can be considered in the first instance
- The DART regime is the most frequently used tapering course of dexamethasone
- Some infants may not respond, and high-dose dexamethasone may be required to facilitate extubation
Contraindications:
- Age < 7 days
- Concomitant NSAID use
- Persistent hypertension, ventricular hypertrophy or hyperglycaemia – may need to consider a more rapid dose wean
2019 European consensus
A short tapering course of low dose or very low dexamethasone should be considered to facilitate extubation in babies who remain on mechanical ventilation after 1–2 weeks
2019 NICE Guidance
Consider dexamethasone to reduce the risk of CLD for preterm babies who are 8 days or older and still need invasive ventilation for respiratory disease
2017 Cochrane review
Evidence showing both benefits and harms of treatment and limitations of available evidence suggests that it may be prudent to reserve the use of late (age >7 days) corticosteroids for infants who cannot be weaned from mechanical ventilation, and to minimise both dose and duration for any course of treatment.
Post-natal inhaled steroids
Therapy with inhaled or nebulised steroids such as budesonide is thought to reduce rates of CLD without the adverse effects associated with systemic steroids. Trials have compared different types of inhaled steroid and also the direct instillation of steroids endotracheally along with surfactant. Although use is increasing, there is no consensus as to optimal timing of treatment and the most effective dosing regime, therefore practice differs significantly between units.
Recommended Dosing regimen:
- Starting dose: Budesonide 500mcg 12 hourly
- Delivered using nebulised Aerogen system for infants receiving respiratory support via ventilator, CPAP driver or high flow oxygen device.
- Dose weaning dependant on the ability to reduced respiratory support and FiO2 trend.
2019 European consensus
Inhaled budesonide can be considered for infants at very high risk of CLD
2019 NICE guideline
No recommendation on the use of nebulised budesonide in preterm babies requiring respiratory support, because of the lack of evidence.
2017 Cochrane review
There is increasing evidence from the trials reviewed that early administration of inhaled steroids to VLBW neonates is effective in reducing the incidence of death or CLD at 36 weeks'
Abbreviation: BPD: Broncho-Pulmonary dysplasia, CLD- Chronic Lung disease CPAP: Continuous Positive airway pressure, FRC: Functional Residual capacity; LISA: Less invasive surfactant administration, MIST: minimally invasive surfactant treatment, NDI: Neuro-Developmental Impairment; NLS: Neonatal life support; PIE: Pulmonary Interstitial emphysema; RCT: Randomized control trial, RDS: Respiratory distress syndrome.
Definitions:
- Primary CPAP: Initiation and maintenance of CPAP for those preterm infants who are at risk of RDS, after initial period of resuscitation, even before signs of RDS develops.
- Effective CPAP: consistent and effective CPAP is usually delivered by use of bi-Nasal prongs/Nasal mask with heated, humidified gas (CPAP/Flow driver).
- Prophylactic surfactant: Intubation and administration of surfactant to those infants who are at risk of RDS, after initial period of resuscitation, even before signs of RDS develops usually within 10-30mins of life.
- Surfactant treatment: administration of surfactant to those infants who have established RDS. Rescue: usually within12 hours when specific criteria for RDS severity was met. Early use of Surfactant: Usually within 2hrs, those who have signs of/Risk of RDS.
Research Questions & Search strategy: Research questions were developed to define the optimal respiratory management in the preterm population (24+0 weeks to <=32 weeks) to improve both short term and long-term outcomes. We used Systematic review method of literature search.
CPAP at Delivery
Earlier systematic review on prophylactic surfactant showed decreased risk of pneumothorax, a decreased risk of pulmonary interstitial emphysema and a decreased risk of mortality when compared to selective use of surfactant. But there was no routine use of CPAP in the control arm of the studies5.
Four recent major RCTs: SUPPORT (24 wks-27 6/7wks), COIN (25+0/7wks -28 6/7), VON (26-296/7wks), CURPAP (25+0/7wks -28 6/7), showed individually no difference in the primary outcome of death or BPD between the CPAP and intubation groups, but did show a positive short term respiratory outcomes including the need for intubation, days of mechanical ventilation, mechanical ventilation at 7days, and post natal steroids use for BPD in the CPAP group. The overall meta-analysis with 1296 infants in the CPAP group and 1486 in the intubation group showed a significant benefit for the combined outcome of death and/or BPD in the CPAP group (RR 0.91, 0.84 to 0.99, Number needed to treat 25)6. The SUPPORT trial also showed a significant decrease in mortality, [23.9% vs. 32.1%, RR=0.74, (0.57, 0.98), p=0.034] in the immature strata of 24 to 25 6/7 weeks gestation randomized to CPAP. Moreover in the latest Cochrane review it has been shown that prophylactic use of surfactant is associated with higher risk of death /BPD ((RR: 1.12; 95% CI: 1.02–1.24; P <. 05)7.
Limitations of these trails:
- All these trials are done in spontaneously breathing infants.
- Except for SUPPORT study all the trails have enrolled preterms with gestational age of 25 weeks and above. So data for 24 weeks is only from SUPPORT study.
- In all these trails, indications for intubation is different and there are differences in their methodology.
- Different types of CPAP devices were used in these trials.
- Each center has different levels of experience with usage of CPAP.
- 50-75% of infants in these trials got intubated at some point.
With the understanding of these limitations, it is evident from the studies that CPAP is at least as effective/better than intubation in preterm population for prevention BPD or BPD/death.
Rationale for CPAP: CPAP increases the trans-pulmonary pressure and increased thoracic gas volume, thereby maintaining functional residual capacity (FRC) and recruits the collapsed alveoli. This increases surface area for gas exchange, reduces the amount work done by the infant each time and reduces intra-pulmonary shunt. This also triggers the release of surfactant from the pool. In animal studies constant distending pressure was also associated with lung growth.
Types Of CPAP Machine & Interfaces: Infant flow driver, which works on the fluid flip model, is more physiological and shown to reduce the work of breathing and shown to be effective. Use of either short Bi-nasal prongs or nasal mask is the current practice. Discussion about individual CPAP machines/interfaces is beyond the scope of this review.
CPAP initiation and Maintenance:
- CPAP should be started at least 6-7 cmH2O and may be increased by increments 0f 1-2 cm H2O based on the clinical situation (increased WOB, increasing respiratory acidosis, increasing oxygen requirement and increasing apnea) up to the maximum pressure of 10 cmH2
- Each increment should be reviewed by a senior, and if needed by C-X-ray/ blood gas.
- Consider BiPAP beyond CPAP pressure of 8 cmH2
- If pressures > 8 cmH2O are required consider whether intubation is required.
Complications Of CPAP:
- Air leaks (pneumothorax, pneumo-pericardium and pneumo-peritoneum). It is evident from COIN trail that higher CPAP pressures (range of 8-10) may be associated with increased chance of air leaks.
- Nasal excoriation, nasal septal injury (pressure necrosis), bleeding and secondary infection.
- Gastric distension and Feed intolerance, but in many of these RCT, the time to reach full feeds is not different between the groups.
- Whether delay in surfactant treatment may lessen the surfactant effect, is still debatable.
Indication for Intubation.
- Indications for intubation during resuscitation are pragmatic and not based on evidence.
- Cochrane meta-analysis of early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants showed that lower treatment threshold (FIO2<0.45) confers greater advantage in reducing the incidences of air-leak syndromes and BPD; moreover a higher treatment threshold (FIO2 at study > 0.45) was associated with increased risk of PDA8.
- Most of the studies (SUPPORT, VON DRM, CURPAP) in the recent systematic review, defined CPAP failure and considered intubation when Fio2 of 40-50%, Pco2 of 60-65mmHg /8-8.5kPa with pH <7.22 and recurrent/severe apneas.
- This suggests that having threshold of 40-50% to intubate is preferable to later selective surfactant treatment.
- Apart from the issue of oxygenation, worsening respiratory distress, poor respiratory drive and hemodynamic instability could either in combination or even individual factor may be an indication for intubation.
- Low threshold to intubate in preterms with no complete antenatal steroids, preterm infants with lower gestational age (≤26 weeks) and lower birth weight (<1Kg).
- Oxygenation: Persistent Fio2 requirement of >40% to maintain SpO2 in the target range (based on the unit policy) even with optimal CPAP/NIPPV support.
- Recurrent/Severe Apnea (adapted from major RCTs): ≥6 apneas within last 6 hours requiring stimulation/intervention or 1 episode requiring positive pressure ventilation, in spite of optimal CPAP/BiPAP support and optimal caffeine therapy.
- Respiratory Acidosis: see the section “Permissive Hypercapnia”.
- Many a times assessment of respiratory distress is subjective, but use of a standardized scoring system like Silverman-Anderson score (Appendix 1) may help us in uniform objective assessment of respiratory distress.
Permissive Hypercapnia9
- Theoretical benefits of permissive hypercapnia are improved oxygen delivery (Bohr effect), increasing respiratory drive and enhancing host defense.
- By having higher PCO2 targets, the mean airway pressure/PIP required will be less and thereby least of barotrauma.
- Retrospective studies in CDH infants have shown that permissive hypercapnia was associated with improved survival and decreased need for ECMO.
- There are 7 RCT analyzing the outcome of permissive hypercapnia.
- 3 of the RCT (2 of them terminated prematurely) primary research question were about permissive hypercapnia and they showed no difference in any outcome.
- Recent, larger RCTs (COIN, SUPPORT, VON, NeoCosur) the primary objective is to evaluate the outcome of intubation versus CPAP and one of the indications for intubation was Pco2 (8-8.5 kPa with H+ > 65 nmol/l).
- By showing no difference in the primary outcome (BPD/Death), it is clear that Pco2 in high range (8-8.5 kPa with H+ < 65 nmol/l) may be safe.
Ventilation Strategies 10:
- Cochrane review with 12 RCT have shown that use of Volume targeted ventilation resulted in reduction of death or BPD (RR 0.73; 0.57-0.93), reduction in pneumothorax (RR 0.46; 0.25-0.84), days of ventilation (RR ;) and combined outcome of Intra ventricular hemorrhage/Peri-ventricular leukomalacia (RR 0.48 (95% CI 0.28 to 0.84) and helps in rapid weaning of ventilation (auto-weaning)11.
- Assist control/ Patient triggered ventilation is more physiological, decrease the work of breathing by supporting every spontaneous breath (overcome the resistance of small endotracheal tube).
- Other ventilation strategies and High frequency ventilation are beyond the scope of this review.
- Regular use of sedation and muscle relaxants should be avoided.
- Every unit should have standard respiratory support weaning guidelines and extubation criteria.
Predictors of CPAP Failure:
- Based on the systematic review that early surfactant is beneficial and more than 50% of preterm infants fail CPAP, it is essential to identify early those infants who are going to fail CPAP.
- There are few retrospective studies available and all these studies were variable with results to predict CPAP failure12-14.
- As we can expect the consistent factor for prediction of CPAP failure in all these studies were lower gestational age and lower birth weight.
- There is no clear evidence available with respect to Fio2 Requirements and other similar oxygenation criteria (like Pao2/Fio2, a-A Do2, product of CPAP pressure with Fio2) to predict CPAP failure.
Early Surfactant (<2hrs) Vs. Delayed surfactant:
- The meta-analyses with six RCT (4 used animal surfactant and 2 used synthetic surfactant) demonstrate significant reductions in the risk of neonatal mortality (RR 0.84; (0.74 to 0.95); chronic lung disease (RR 0.69; (0.55 to 0.86); and chronic lung disease or death at 36 weeks (RR 0.83; 0.75 to 0.91) associated with early treatment of intubated infants with RDS.
- Intubated infants randomized to early selective surfactant administration also demonstrated a decreased risk of acute lung injury including a decreased risk of pneumothorax (RR 0.69; 0.59 to 0.82;), pulmonary interstitial emphysema (RR 0.60; 0.41 to 0.89) and overall air leak syndromes (RR 0.61; 0.48 to 0.78)7.
Uses of surfactant:
- It is one of the well-studied drug therapies in neonatal medicine, which improved neonatal survival.
- Short term: improves Functional Residual capacity (FRC) and surface area for oxygenation and decreases the barotrauma.
- Systematic review on Surfactant use in established RDS have shown to reduce mortality, air leaks (pneumothorax, PIE), and BPD (at 28 days) /death 15. This surfactant therapy is more effective in preterms <30 wks. Birth wt. <1250g16.
Surfactant administration:
- To avoid uneven distribution of surfactant to one side of lung, consider confirmation of ETT position by chest X-ray, prior to surfactant administration.
- Large volumes, given as single bolus of surfactant will have better distribution than smaller dose/multiple aliquots distribution17,18.
- There is limited data regarding benefits with maintenance of PEEP during surfactant administration (surfactant through side port adaptor).
- Until further optimum methods are described surfactant should be administrated as single bolus with largest dose possible and administered either through main port/side port as per the unit policy.
Post Surfactant Management:
- There is no clear evidence to answer whether mechanical ventilation or PPV with neopuff/ Flow inflating bag is better after the surfactant administration.
- Desaturation and bradycardia is a frequent event in all the clinical trails, post surfactant administration.
- If the desaturation/bradycardia is not improving consider increasing the PIP and/or Fio2.
- Once the surfactant is administered great care is needed to wean the ventilator settings as the compliance improves very rapidly.
Types of surfactant.
- With widespread use of curosurf in UK and proven clinical benefits (systematic review) of reduced mortality rates, reduced need for re-dosing and initial respiratory support19, it is recommended to use curosurf (at a dose of 200mg/kg).
Repeat Doses Of Surfactant.
- Single treatment dose of 100-200mg/kg surfactant is very large relative to the surfactant pool of preterm animal (>4mg/kg)20.
- With long half life of surfactant of 12hrs, repeat dose of surfactant is unlikely to be needed, unless there is surfactant inactivation by blood /meconium.
- Many of the trials had scheduled doses of surfactant rather than based on clinical presentation like residual RDS. Those trials that used repeat doses of surfactant, many responded, few of them didn’t show any response probably due to underlying conditions like Pneumonia/pulmonary hypoplasia 21,22.
- In the recent era of higher antenatal steroid coverage, primary CPAP there are no prospective clinical trials for repeat dose of surfactant.
- Repeat dose of surfactant should be individualized based on the residual lung disease which if not treated could lead to complications like prolonged ventilation/pneumothorax.
- Isayama T, Chai-Adisaksopha C, McDonald SD. Noninvasive Ventilation With vs Without Early Surfactant to Prevent Chronic Lung Disease in Preterm Infants: A Systematic Review and Meta-analysis. JAMA Pediatr 2015.
- Majnemer A, Riley P, Shevell M, Birnbaum R, Greenstone H, Coates AL. Severe bronchopulmonary dysplasia increases risk for later neurological and motor sequelae in preterm survivors. Dev Med Child Neurol 2000;42(1):53-60.
- Singer L, Yamashita T, Lilien L, Collin M, Baley J. A longitudinal study of developmental outcome of infants with bronchopulmonary dysplasia and very low birth weight. Pediatrics 1997;100(6):987-93.
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D and others. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490.
- Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2001(2):CD000510.
- Schmolzer GM, Kumar M, Pichler G, Aziz K, O'Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. Bmj 2013;347:f5980.
- Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev 2012;11:CD001456.
- Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 2007(4):CD003063.
- Ryu J, Haddad G, Carlo WA. Clinical effectiveness and safety of permissive hypercapnia. Clin Perinatol 2012;39(3):603-12.
- Wheeler K, Klingenberg C, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in the neonate. Cochrane Database Syst Rev 2010(11):CD003666.
- Sant'Anna GM, Keszler M. Developing a neonatal unit ventilation protocol for the preterm baby. Early Hum Dev 2012;88(12):925-9.
- Ammari A, Suri M, Milisavljevic V, Sahni R, Bateman D, Sanocka U, Ruzal-Shapiro C, Wung JT, Polin RA. Variables associated with the early failure of nasal CPAP in very low birth weight infants. J Pediatr 2005;147(3):341-7.
- Fuchs H, Lindner W, Leiprecht A, Mendler MR, Hummler HD. Predictors of early nasal CPAP failure and effects of various intubation criteria on the rate of mechanical ventilation in preterm infants of <29 weeks gestational age. Arch Dis Child Fetal Neonatal Ed 2011;96(5):F343-7.
- Dargaville PA, Aiyappan A, De Paoli AG, Dalton RG, Kuschel CA, Kamlin CO, Orsini F, Carlin JB, Davis PG. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology 2013;104(1):8-14.
- Polin RA, Carlo WA. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics 2014;133(1):156-63.
- Suresh GK, Soll RF. Overview of surfactant replacement trials. J Perinatol 2005;25 Suppl 2:S40-4.
- Segerer H, van Gelder W, Angenent FW, van Woerkens LJ, Curstedt T, Obladen M, Lachmann B. Pulmonary distribution and efficacy of exogenous surfactant in lung-lavaged rabbits are influenced by the instillation technique. Pediatr Res 1993;34(4):490-4.
- Espinosa FF, Kamm RD. Bolus dispersal through the lungs in surfactant replacement therapy. J Appl Physiol (1985) 1999;86(1):391-410.
- Singh N, Hawley KL, Viswanathan K. Efficacy of porcine versus bovine surfactants for preterm newborns with respiratory distress syndrome: systematic review and meta-analysis. Pediatrics 2011;128(6):e1588-95.
- Jobe AH. Pulmonary surfactant therapy. N Engl J Med 1993;328(12):861-8.
- Hoekstra RE, Jackson JC, Myers TF, Frantz ID, 3rd, Stern ME, Powers WF, Maurer M, Raye JR, Carrier ST, Gunkel JH and others. Improved neonatal survival following multiple doses of bovine surfactant in very premature neonates at risk for respiratory distress syndrome. Pediatrics 1991;88(1):10-8.
- Liechty EA, Donovan E, Purohit D, Gilhooly J, Feldman B, Noguchi A, Denson SE, Sehgal SS, Gross I, Stevens D and others. Reduction of neonatal mortality after multiple doses of bovine surfactant in low birth weight neonates with respiratory distress syndrome. Pediatrics 1991;88(1):19-28.
Last reviewed: 15 November 2021
Next review: 01 November 2024
Author(s): Dr Joyce O’Shea - Consultant Neonatologist, NHS Greater Glasgow & Clyde; Dr Sandy Kirolos – Neonatal Trainee NHS Greater Glasgow & Clyde
Co-Author(s): Acknowledgement of work on previous versions: Dr Prakash Loganathan, - Consultant Neonatologist, NHS Greater Glasgow & Clyde; Dr Vrinda Nair - Consultant Neonatologist, NHS Ayrshire & Arran; Other professionals consulted: June Grant – Pharmacist, NHS Greater Glasgow & Clyde
Approved By: West of Scotland Neonatology Managed Clinical Network
Document Id: 966

