ABPA and aspergillus lung infection, cystic fibrosis patients RHC
exp date isn't null, but text field is
Objectives
Guidance for clinicians and healthcare staff on the diagnosis and treatment of Allergic bronchopulmonary aspergillosis
Scope
Patients under the care of the paediatric cystic fibrosis unit RHC Glasgow
Audience
Disclaimer: The following guideline has been developed for use within the Royal Hospital for Children, NHS Greater Glasgow and Clyde (NHSGCCC). The guideline has been developed in collaboration with key stakeholders within NHSGGC, including Microbiology, Cystic Fibrosis, Infectious Disease and Pharmacy teams. The guideline has been approved by the Paediatric Antimicrobial Management Team. This guideline does not account for epidemiology and resistance patterns outside of NHS GGC and use outside of the designated organisation is at the individual’s risk.
Allergic bronchopulmonary aspergillosis (ABPA) and aspergillus lung infection in paediatric cystic fibrosis patients – diagnosis and management RHC CF Unit Glasgow
Allergic bronchopulmonary aspergillosis (ABPA) is a complex respiratory disorder characterised by a hypersensitivity reaction to Aspergillus fumigatus. It affects approximately 10% of patients with cystic fibrosis (CF). Untreated, inflammation may result in bronchiectasis, fibrosis and eventually loss of lung function. ABPA can be difficult to diagnose. Diagnostic criteria require an evaluation of clinical and radiological signs, lung function trend and serum immunologic markers such as total Ig E , Aspergillus IgE and Aspergillus IgG.
DIAGNOSIS
Early diagnosis and treatment are important in preventing the complications associated with ABPA. Early diagnosis depends on a combination of screening and high clinical suspicion. Atypical cases may lack some or all of the diagnostic criteria – maintain a high index of suspicion an discuss with the Consultant.
Bloods – Positive ABPA serology plus at least one other marker suggestive of ABPA
- Total IgE > 450 kU/L
- Aspergillus. Specific IgE > 0.35 kU/L
- Aspergillus. Specific IgG > 90 mgA/L
- Eosinophils > 0.4 (x109g/L)
Sputum / Cough Swabs
Aspergillus fumigatus may or may not be present in respiratory cultures.
Clinical features for consideration
- Clinical signs consistent with pulmonary infection, particularly if failing to respond to antibiotics and inhaled medications
- Fever/malaise
- Change in pulmonary function/reduction in FEV1
Other investigations
- Chest X-ray may be of use. Evidence of pulmonary infiltrates, segmental collapse or central bronchiectasis may be indicative of ABPA.
ABPA - TREATMENT
Treatment of ABPA includes a combination of oral Prednisolone plus an oral anti-fungal agent.
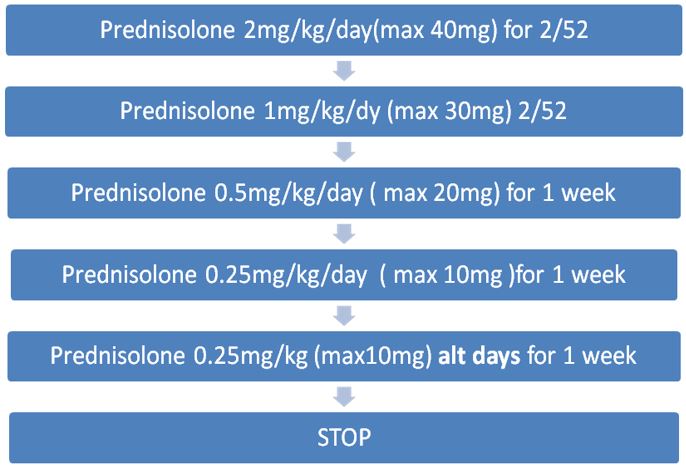
CORTICOSTEROIDS
Oral corticosteroids are given to reduce inflammation and suppress immune hyper-reactivity. Use is based largely on clinical experience due to a lack of randomised controlled trials. The following dosing regimen is based on consensus from the Cystic Fibrosis Foundation.
At RHC, prednisolone is the oral steroid of choice for ABPA.
Oral prednisolone should be given in the morning after food. Enteric coated preparations should be avoided as these are not well absorbed in CF.
Varicella antibody (IgG) should be checked prior to starting steroids for ABPA. If negative, caution patient/parent to be vigilant for possible chicken pox exposure during steroid treatment.
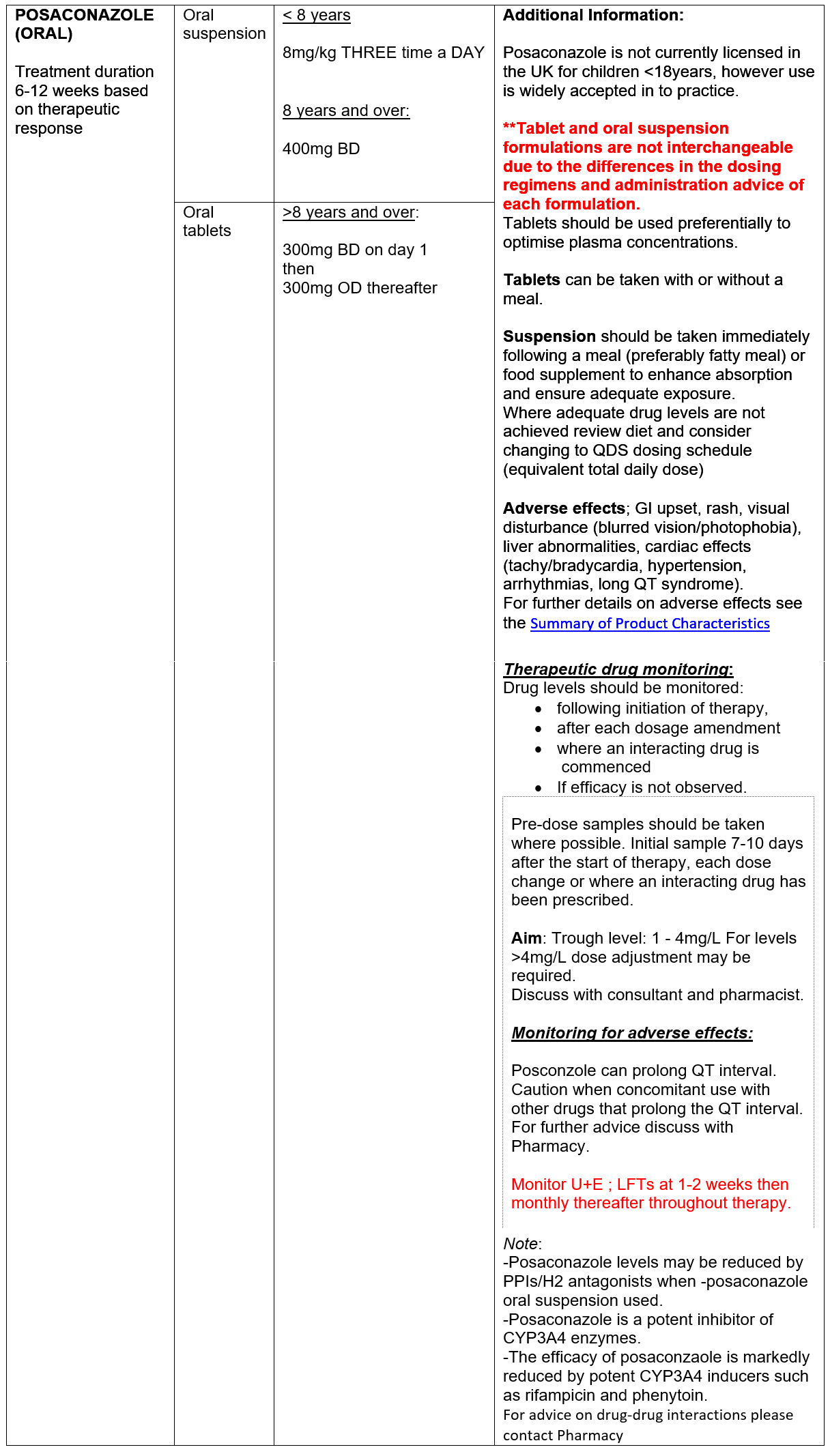
ANTIFUNGAL TREATMENT
Oral ‘azole’ antifungals (AFs) are used to reduce fungal burden in the airways to attenuate immune response. Increasing data supporting the use of the ‘newer’ antifungal agents, posaconzole and isavuconazole, in CF patient with ABPA is emerging. Neither agent is currently licensed for use in children <18 years in the UK, however use has been widely accepted in to clinical practice. Posaconazole and isavuconazole have favourable side effect profiles compared to voriconazole. In addition, the newer agents have a more predictable kinetic profiles with less inter-patient variability compared with voriconazole, better facilitating therapeutic monitoring and management of drug-drug interactions.
Azole antifungal agents inhibit CYP3A4 enzymes pathways with varying degrees of potency, resulting in increased exposure to CYP3A4 substrates including Ivacaftor (Kalydeco), tezacaftor/ivacaftor (Symkevi) and elexacaftor/tezacaftor/ivacaftor (Kaftrio). Dose adjustment of the CFTR modulators is required. Interactions with lumacaftor/ivacaftor (Orkambi) are more complex - contact Pharmacy for further advice.
Failure to respond to initial therapy:
Where clinical response is poor,
- Consider IV methylprednisolone in place of oral steroids – discuss with Consultant and Pharmacy.
- Check serum azole level is therapeutic. If not review adherence and consider dose increase before switching treatment.
- Check for any newly initiated therapy and potential drug-drug interactions.
- Consider switch in antifungal therapy, taking note of any sensitivities available. Discuss with Microbiology and Pharmacy for dosing advice and in to review drug-drug interactions.
Relapse/hard to treat ABPA/frequently relapsing ABPA
Relapse is relatively common and can occur months to years after the first episode. Repeat treatment courses will be required. Changes from initial therapy should be considered – discuss with Microbiology.
All cases of relapse or difficult to treat ABPA should be discussed with the CF MDT, including Microbiology. Alternative treatment strategies may be considered, including:
- Nebulised amphotericin (non-liposomal) for up to 3 months
- Isavuconazole
- IV caspofungin
- Omalizumab – requires ULM application.
Use of voriconazole is should be avoided where possible due to variable kinetic profiles (DDIs) and limiting side effects. The MHRA published a drug safety update highlighting the risk of liver toxicity and squamous cell carcinoma following phototoxic reactions. Where voriconazole use is unavoidable, MDT discussion is required.
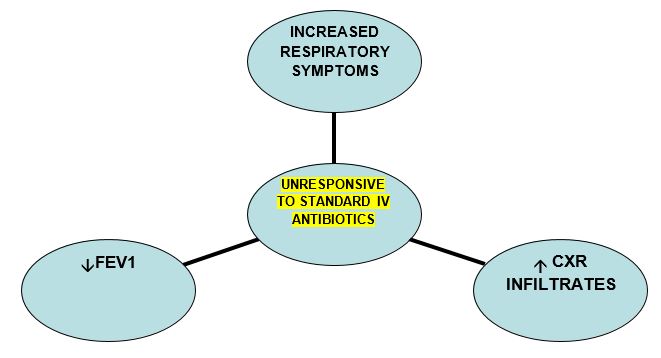
DIAGNOSIS
This is a relatively rare complication in CF. Diagnosis may be suspected if some of the following criteria are met:
- Aspergillus in sputum
- Worsening CXR infiltrates +/- cavitation and nodules
- Unresponsive to standard IV antibiotic treatment
- Elevated serum Galactomannan ( Asp.fumigatus cell wall component)
- +/- Elevated Aspergillus fumigates IgE/ IgG
TREATMENT
If pulmonary infection is believed to be due to Aspergillus species a 2 week course of IV antifungal followed by oral antifungal treatment may be considered.
Posaconazole should be considered the first line agent for treatment of Aspergillus Lung Infection in CF.
Intravenous therapy should always be discussed with a Consultant Microbiologist and the CF MDT.
IV Posaconazole may be considered. It is not licensed for use in children however limited dosing information is available. IV posaconazole contains SBECD as a solubilizing excipient. Please contact Antimicrobial/CF Pharmacist for advice.
PO Posaconazole – for dosing & monitoring advice please see section on ABPA
IV Voriconazole is reserved as a second-line agent due to the variable and unpredictable pharmacokinetic profile, side effect profile and significant drug-drug interaction with CFTR modulator agents. Dose reduction of CFTR modulators are required and it may be necessary to stop the drug completely during treatment with voriconazole.
Voriconazole doses are as per BNF-c/SmPC. Therapeutic drug monitoring is required, however samples are only processed on a Wednesday in NHS GGC. This should be considered when planning IV treatment.
Serologic diagnosis of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis through the detection of immunoglobulin G to Aspergillus fumigatus R.C. Barton et al. / Diagnostic Microbiology and Infectious Disease 62 (2008) 287–291
Aspergillus bronchitis in cystic fibrosis Shoseyov D, Brownlee KG, Conway SP, Kerem E. Chest 2006;130:222–6.
Clinical Guidelines:Care of Children with Cystic Fibrosis Royal Brompton Hospital 2014/5
CF Trust “ Antibiotic Treatment for Cystic Fibrosis ” 2009 Section 7.9 ABPA
Pulmonary aspergillosis: a clinical review M. Kousha, R. Tadi and A.O. Soubani Eur Respir Rev 2011; 20: 121, 156–174
Last reviewed: 27 November 2023
Next review: 30 June 2026
Author(s): Dr Louise Thomson
Version: 2
Co-Author(s): Dr Anne Devenny, Dr Christine Peters, Dr Patrick McCrossan, Ms Susan Kafka
Approved By: Paediatric Drugs & Therapeutics Committee

