New psychoactive substances (NPS): Emergency Department guidance
exp date isn't null, but text field is
Objectives
Management advice for patients presenting with the effects of New Psychoactive Substances (NPS) to the Emergency Department. This includes the management of serotonin toxicity and the intoxicated child/adolescent flowchart to aid in discharge management.
Scope
Children and adolescents presenting to the Emergency Department after taking an NPS.
Audience
Emergency Department nursing and medical staff.
New Psychoactive Substances, formerly known as ‘legal highs’, are substances that are produced to mimic the effects of traditional recreational drugs. Since 2008 the presence of these drugs in our communities has been increasing. As of November 2018, over 700 substances are being monitored by the European Monitoring Centre for Drugs and Drug Addiction [1]. The Novel Psychoactive Substances Act, introduced in 2016, means that it is now illegal to sell Novel Psychoactive Substances, therefore most are bought online. They are often labelled as ‘Research Chemicals,’ ‘Herbal Incense, ‘Bath Salts’ or ‘Plant Food’ and are often labelled ‘Not for human consumption.’ New substances quickly replace banned substances.
Younger people increasingly use these drugs and multiple substances are often taken simultaneously. The chemicals in the drug often differ from the written description and the composition of one product may vary from month to month. This makes the psychoactive and toxicological effects of these drugs highly unpredictable in the short term, with unknown long-term effects. Most have never been tested on humans in a controlled environment therefore our knowledge about these substances is very limited. We are slowly acquiring more information about the toxidromes of certain drugs as patients present to Emergency Departments.
These substances present significant diagnostic and management problems to Emergency Departments in individual cases, however, we know that groups may share drugs. Multiple patients may therefore present to Emergency Departments simultaneously with toxic effects of NPS, as was seen at Glasgow Royal Infirmary in 2013.
In Scotland in 2013 there were 113 deaths where NPS were present and a 16 year old died in Lankshire in August 2014 after taking an NPS.
The most commonly used groups of NPS in the UK are:
- Synthetic Cannabinoids (dissociative type)
- Stimulant-type NPS
- Benzodiazepine-type NPS (sedative type)
Below is a description of the effects and side effects of the more common NPS in the UK. It is important to remember that the market for these drugs is constantly changing and patients may be unaware or unable to tell you what they have taken. You must therefore treat each toxidrome as you find it (eg; hypotension, arrhythmias, seizures, pyrexia), whilst maintaining a broad differential diagnosis (sepsis may present in a similar manner).
Adverse effects of illicit drups, including NPSs can be reported to the Report Illicit Drug Reactions (RIDR) pilot project. Details are on toxbase.
(eg: Bombay Blue, Annihilation, Clockwork Orange, Doctor Green Thumb, Exodus, Herbal Haze, Joker, Sensate, Pineapple Express, Spice, Herbal Incense)
These chemicals mimic the effects, and are often more potent than, the active component of cannabis (THC).
Method of Ingestion: Usually smoked but can be ingested
Desired effects: Similar to cannabis - relaxation, euphoria and dissociative state
Side Effects:
- CNS: Anxiety, hallucinations, change in perception, nystagmus, acute psychosis, convulsions, coma, hypertonia and myoclonus
- GI: Nausea, vomiting
- CVS: tachycardia, tachyarrhythmias, palpitations and chest pain, QTc prolongation and syncope
- Other: Cold extremities, dry mouth, dyspnoea, mydriasis and hypokalaemia
- Toxic effects generally last up to 4 hours, however, as with all NPS the effects are unpredictable and may last considerably longer
Management: See Toxbase ‘Synthetic Cannabinoid Receptor Agonists’
- Post Ingestion – 6 hours observation
- Post Inhalation – Asymptomatic patients do not require observations
- In symptomatic patients monitor heart rate and BP. Perform 12-lead ECG and take blood glucose measurement (BM)
- It is better to manage agitation without sedation. (Parents present. Nurse in quiet lowered light environment).
- Midazolam 0.05-0.1mg/kg if necessary for agitation.
- Manage seizures normally. Use Lorazepam 0.1mg/kg if required.
Synthetics Cathinones & Phenylamines (Gogaine, Ching, Mr. White, Columbiana, Charly Sheen, Dust till Dawn, Ivory Wave, Blue Stuff, Bath Salts, Sniff)
Mephedrone (Magic, Bubbles, M-Cat, Meow Meow, Plant Food)
Method of Ingestion: Snorted as a powder, ingested in tablet form, ingested as powder wrapped in cigarette paper (‘bombing’), or injected
Desired Effects: Similar to that of amphetamines, cocaine and MDMA – euphoria, increased alertness, intensified emotions and boosted self-esteem
Side Effects:
- CNS: Tremor, sweating, dilated pupils, agitation, confusion, headache, anxiety, seizures, hallucinations or delusions.
- CVS: Chest pain, palpitations, dyspnoea, hypotension or hypertension may occur. Narrow-complex tachycardias are common; ventricular tachycardia or ventricular fibrillation may occur. Coronary Ischaemia
- GI: vomiting, abdominal pain
- Hyperpyrexia may be severe.
- Metabolic acidosis may also occur.
- Effects may have duration of 24-48 hours.
Management : See Toxbase under specific drug or ‘Amfetamines and other stimulants’ and Serotonin Toxicity
- Activated Charcoal if within 1 hour of ingestion.
- Observations - at least 4 hours. 30 minutely observations (HR, Rhythm, BP, GCS and To)
- ECG - Serial ECGs may be required in symptomatic patients
- Measure U&Es, LFTs, CK and Coag if features of toxicity are present.
- Agitation and delirium - Midazolam 0.05-0.1mg/kg if required
- Seizures – Normal management. Lorazepam 0.1mg/kg or Midazolam 0.1-0.15mg/kg
- Metabolic Acidosis – Correct any hypoxia and hypovolaemia. Consider 8.4% Soduim Bicarbonate
- Narrow Complex Tachycardias - best left untreated in uncompromised patients
- Hypotension – IV fluids. Discuss with ICU if hypotension unresponsive to fluids
- Hyperthermia – Aggressively cool patients. (See Serotonin Toxicity)
If Hyperthermia refer to Serotoxin Toxicity Guidance below
(Eg: Phenazepam, Etizolam, Diclazepam, Flubromazolam, Flubromazepam)
The primary toxicity of benzodiazepines ins CNS depression. These Benzodiazepinetype NPS may be many times more potent that Diazepam.
Desired Effects: Same as Diazepam - sedation, anxiolysis, hypnosis.
Side Effects:
- CNS: Drowsiness, ataxia, dysarthria and nystagmus, coma
- CVS: Bradycardia, Hypotension
- ECG Abnormalaties: 1nd/2nd degree block and QTc Prolongation
- Respiratory: Respiratory Depression
Management - see Toxbase under specific drug or drug class
- Consider Charcoal if <1 hr since ingestions
- Monitor Vital signs - will require at least 4 hours of observation post exposure
- Perform ECG
Flumazenil is an appropriate treatment in patients poisoned only by benzodiazepines who develop reduced ventilation and coma who would otherwise require mechanical ventilation. It should not be used as a diagnostic test and should not be use in patients who have co-ingested medicines that may cause seizures. (See toxbase)
Serotonin toxicity is a serious side effect of ingestion of NPS. Features may occur insidiously over a period of hours to minutes and are characterized by altered mental state, neuromuscular hyperactivity and autonomic instability.
- Altered Mental State – Agitation, confusion, delirium, hallucinations, drowsiness and coma
- Neuromuscular Hyperactivity – shivering, tremor, teeth grinding, myoclonus and hyperreflexia
- Autonomic Instability – Tachycardia, fever, hyper- or hypotension, flushing, diarrhoea and vomiting
In severe toxicity, uncontrolled hyperpyrexia leads to rhabdomyolysis, renal failure, acidosis, hypocalcaemia, hyperkalaemia, DIC and death. It is therefore paramount that it is diagnosed and treated early.
This flow chart can help aid diagnosis of Serotonin Toxicity (Formed from the Hunter Serotonin Toxicity Criteria (Sensitivity 84%, specificity 97%):
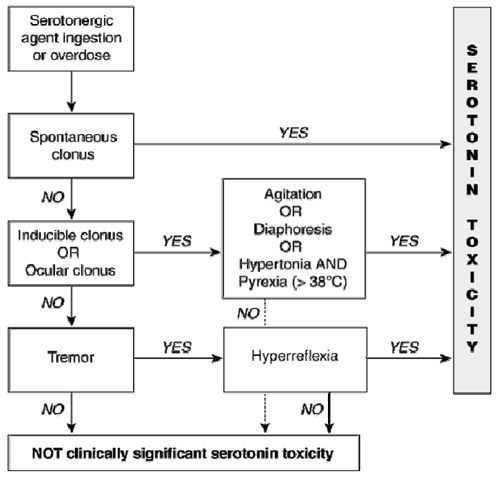
Serotonin Toxicity
*There is no evidence to support the use of Dantrolene effectivement in serotonin toxic patients.
Investigations: (Dictated to by presentation)
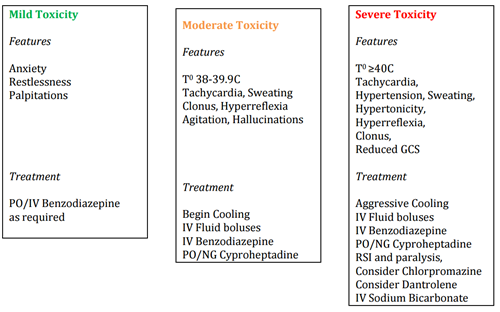
Mild toxicity - U&Es, CK, Glucose, VBG, ECG
Moderate / severe toxicity - U&Es, FBC, LFTS, Ca2+, Coag, CK, VBG, Glucose, ECG
Role of urine toxicology - Helpful for surveillance of agents only. Does not change management as most NPS NOT detected.
Cooling:
- Cooled IV fluids and cooled fluids for bladder lavage
- Ice - applied by packing axilla and groin with ice
- Antipyretics have no role for serotonin toxicity induced hyperpyrexia
- Bair Hugger - on cool
RSI
Opiate - avoid Fentanyl/Alfentanil due to serotonergic action
Recommended induction agent:
- Thiopentone 3-5mg/kg (Avoid Ketamine)
Muscle relaxant - Rocuronium 1mg/kg for induction
Depolarising neuromuscular blockers (Suxamethonium) are contraindicated
Hyperkalaemia
- 0.5ml/kg Calcium Gluconate 10%
- Insulin/Dextrose infusion if blood sugar levels normal
Rhabdomyolysis
- IV fluids
- Consider 3-5 mmol/kg of Sodium Bicarbonate (3-5mls/kg of 8.4% NaBic)
Drug Doses
- Midazolam: 0.05-0.1mg/kg IV
- Lorazepam: 0.1mg/kg (4mg max) IV
- Cyproheptadine: PO or crushed and administered via NG
Adults and children aged 13 or more: 12 mg orally, followed by 4-8mg every 6hrs
Children aged 12 years or less: 0.25mg/kg/day (max 12mg) in 4 divided doses - Chlorpromazine: 500mcg/kg IM 6-12 years, 25-50mg IM 12-18 years
- Dantrolene*: 2-3mg/kg IV
* There is no evidence to support the use of Dantrolene effectiveness in serotonin toxic patients.
Important points about management of serotonin toxicity management:
- Mortality is significantly raised in patients with a temperature >40oC
- Cyproheptadine and chlorpromazine are unlicenced for this use. Their use treats symptoms but as yet do not alter mortality.
- Acute kidney injury, liver failure, disseminated intravascular coagulation, rhabdomyolysis are complications associated with moderate to severe serotonin toxicity.
- Toxicology studies of NPS are extremely limited.
- NPS have a greater duration of effect than ecstasy.
- EMCDDA - Early Warning System on NPS.
- Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med J Aust 2007; 187:361-365
Last reviewed: 03 September 2019
Next review: 31 October 2025
Author(s): Dr Steven Rainey (Emergency Medicine Consultant, Glasgow Royal Infirmary)
Version: 3
Co-Author(s): Dr Richard Stevenson, (Emergency Medicine Consultant, Glasgow Royal Infirmary); Dr Siobhan Sweeney (Paediatric Emergency Medicine Consultant, RHCG); Correspondence author: Dr Steve Foster (Paediatric Emergency Medicine Consultant, RHCG).
Approved By: Clinical Effectiveness
Reviewer Name(s): Paediatric Clinical Effectiveness & Risk Committee

