Surfactant Administration via Laryngeal Mask Airway (LMA) Standard Operating Procedure
exp date isn't null, but text field is
Objectives
This Standard Operating Procedure is intended to ensure that practitioners administering surfactant via a Laryngeal Mask Airway (LMA) do so in a standardised manner to maintain safe practice. The decision to administer surfactant using this technique is at the discretion of the attending neonatal consultant. Anyone undertaking this procedure should ensure that they have received instruction from a senior clinician who is experienced in the technique.
Preterm infants commonly develop respiratory distress syndrome (RDS) requiring some form of respiratory support and potentially surfactant administration (1). Treatment with surfactant has been shown to reduce the risk of death and bronchopulmonary dysplasia (BPD) in preterm infants; however, the standard approach to administering surfactant involves using an endo-tracheal tube and a period of mechanical ventilation (MV). It is well known that MV causes damage to the fragile preterm lungs (2) so many lung protective strategies for respiratory management and ventilation have been developed.
As per our current guideline on the respiratory management of preterm infants the preferred initial management is using primary CPAP with the use of rescue surfactant for infants with an increasing oxygen requirement. To reduce the need for MV but still deliver surfactant several less invasive techniques have been developed.
The successful use of the laryngeal mask to deliver surfactant in preterm infants with RDS has been described in randomised controlled trials since 2013 (3)(4)(5). More recently Roberts et al described a significant reduction in the requirement for mechanical ventilation in preterm infants with moderate RDS who received surfactant by LMA compared with infants maintained on primary CPAP (6).
Use of the laryngeal mask airway is increasing in the neonatal population with use described in infants as small as 800g (7) and routinely in infants of 1200g.
The literature supports use of this technique in infant’s ≥1200g and ≥28 weeks gestation (3-6). However, it is worth noting that the smallest LMA available are marketed for use at weights of 2-5Kg.
For the purpose of this guideline it is suggested that surfactant administration by LMA is considered in infants born ≥28 weeks gestation weighing ≥1200g.
Infants who are suitable to be considered for surfactant administration by LMA are those being managed with primary CPAP with clinical and radiological evidence of RDS and with a rising oxygen requirement. If an infant is commenced primarily on High Flow Nasal Cannulae and develops worsening respiratory distress with rising oxygen requirement, they should be converted to nasal CPAP and observed for at least 30 minutes.
A threshold of a sustained FiO2 of 30% or more in the first 48 hours is suggested for infants <32 weeks gestation. In addition, surfactant administration by LMA can be considered in more mature infants (32-36 weeks gestation) at the higher oxygen threshold of 30-35% FiO2.
One of the advantages of this technique is that it is a relatively simple procedure to perform compared with other routes of surfactant administration. Despite this it should be performed in the controlled environment of the neonatal unit where full monitoring and equipment are available.
- Imminent need for intubation as judged clinically by the attending senior clinician
- Maxillo-facial, tracheal or known pulmonary malformations
- Major congenital abnormalities including confirmed or suspected congenital cardiac disease
- Alternative cause for respiratory distress e.g. Congenital pneumonia
- Resuscitation T-Piece Circuit with Duckbill Port connected to the neopuff
- i-gel size 1
- lubricating jelly
- surfactant giving set
- pedicap
- appropriately sized face mask
- suction
- NG tube (to insert after procedure)
- Sucrose
- Curosurf® 200mg/kg to the nearest vial drawn up into a 5 ml syringe. The maximum dose which can be given in any one instillation is limited to 480mg. This may result in larger babies receiving less than 200mg/kg.
- Atropine – use prophylactically in all infants prior to insertion of the i-gel
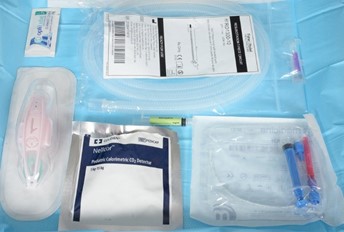
Equipment preparation (see appendix 1 for images)
- Ensure duck bill port is patent and that the surfactant giving set catheter passes easily. If this is not the case, replace duck bill port set.
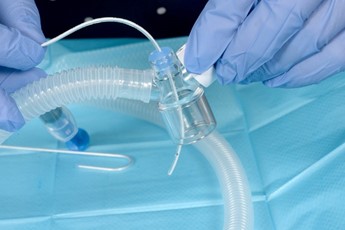
- Connect the T piece circuit with duck bill port to the neopuff. The T Piece with duck bill port can be used in the standard way to provide both PEEP and IPPV
- Set PIP, PEEP and O2 as required. PEEP and FiO2 should be the same as is required to maintain stability on CPAP
- Ensure suction working
- Draw up required volume of surfactant and lubricate tip of catheter with lubricating jelly
- Remove I-Gel from packaging and ensure liberal use of lubricating gel on the posterior aspect and sides
Evidence suggests that this procedure is well tolerated and sedating medications are not required.
Atropine;
- administered routinely prior to the procedure. See formulary for dosing information.
If the procedure has to be abandoned for clinical reasons it is likely the infant can be supported with mask ventilation or a return to non-invasive ventilation. All the standard equipment and medications required for intubation in case of emergency should be available.
Preparing the Infant (see appendix 1 for images)
Infants undergoing this procedure should have continuous monitoring of heart rate and oxygen saturations. Secure intravenous access must be confirmed prior to commencing the procedure. Measures to maintain normothermia should be taken.
The infant should be maintained on non invasive respiratory support (CPAP) throughout the procedure. It may be necessary to move the mask or prongs out of position after insertion of the LMA. These should be replaced prior to removal of the LMA to maintain effective CPAP throughout.
The stomach should be emptied of contents and air immediately prior to the procedure. The gastric tube should be removed immediately before the LMA is placed and a new gastric tube should be available for placement immediately following the procedure.
The baby can be positioned as for intubation or remain supine inline with the incubator. The head should be maintained in the midline throughout.
Administer atropine.
- Lubricate the back and sides of the iGel
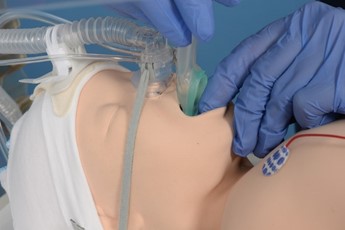
- Tilt the babies head back slightly, open the mouth and apply jaw thrust
- Insert the tip of the iGel along the hard palate with the open side facing the tongue
- Continue inserting the LMA/iGel along the posterior pharyngeal wall. Resistance is felt when the LMA/iGel tip sits on the oesophagus
- The opening of the mask should cover the entrance to the larynx (See figure 1).
- In some infants, a tongue depressor/laryngoscope may be required to maintain tongue position while inserting the LMA.
Figure 1: The final position of the iGel in the airway
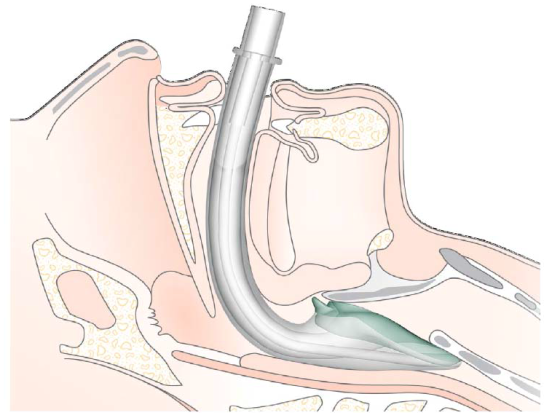
(see appendix 1 for images)
Surfactant administration by LMA should be undertaken by members of staff who are familiar and trained in the procedure.
This includes registrars who have been previously supervised and assessed to be competent. One of the advantages of this technique is that it does not require the same degree of skill as intubation or LISA.
A pre-procedure pause should be undertaken to ensure all members of the team understand their roles Appendix 2.
The LMA needs to be inserted and held in place by the member of the team responsible for airway control during the procedure. A second person should then carry out all additional manoeuvres including attaching the circuit, passing the surfactant catheter and giving the surfactant
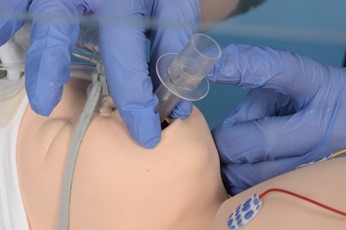
- Insert the well lubricated LMA as described
- Attach pedicap and T piece ensuring appropriate PEEP is delivered
- Confirm placement using pedicap (repositioning if required)
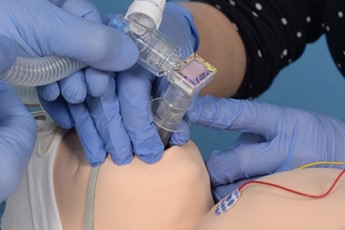
- Remove pedicap and connect T piece directly to LMA
- Ensure observations stable and any transient bradycardia has resolved. A period of bradycardia with or without desaturation and apnoea can be anticipated during insertion and removal of the LMA. This will often resolve spontaneously.
- If required IPPV and additional oxygen can be delivered through the T-piece. Brief IPPV can often resolve episodes of prolonged bradycardia or apnoea. Desaturation can be anticipated during instillation of surfactant and can be treated by increasing oxygen as required during the procedure
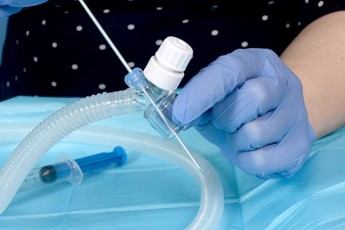
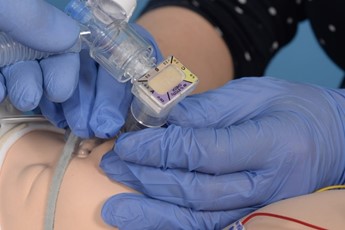
- Pass the surfactant catheter through the duck bill port and into the LMA to a depth of 14-16cm. There may be some resistance as the tip of the catheter nears the end of the LMA. This can be overcome with gentle manipulation or injecting a small amount of surfactant to lubricate the tip of the giving set.
- Give the required volume of surfactant over 2-5 minutes as tolerated. It is better to pause if the infant has an episode of bradycardia, apnoea or desaturation and allow spontaneous resolution or give a period of IPPV and/or increased oxygen through the LMA if required than to rush the procedure and give the surfactant too quickly.
- Remove the surfactant giving set
- Ensure the CPAP mask or prongs are in place prior to removing the LMA
- Reconnect the standard neopuff circuit
It is important to take a note of the final volume of Surfactant administered in mls. There is evidence to suggest that if more than 50% of the administered dose of Surfactant is aspirated from the stomach post procedure the risk of subsequent intubation and ventilation increases (6). These infants should be closely monitored and consideration given to additional surfactant by LMA or ETT based on clinical status.
Continue on non-invasive ventilation weaning oxygen and PEEP as tolerated. If the procedure has been successful an improvement in respiratory distress and oxygen requirement can be anticipated.
Pass a gastric tube as soon as possible and aspirate contents, document any residual Surfactant obtained
There is no requirement for repeat CXR if the infant is stable and has clinically improved.
If there is deterioration or no clinical improvement 2 hours after the procedure, the infant should be reassessed and consideration given for a further dose of Surfactant. This can be given in the traditional way via and ET tube following intubation. Repeat LMA surfactant is not currently advised while the technique is still evolving.
All infants who undergo this procedure should have a data collection form completed and submitted (appendix 3).
 |
 |
|
Equipment required |
|
 |
 |
|
Prepare the Duckbill Port by passing the surfactant catheter or stylet to ensure it is patent |
|
 |
 |
|
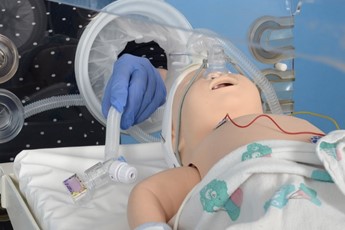
Connect circuit to neopuff, set PEEP, PIP and oxygen |
Attach pedicap and place in incubator |
 |
 |
|
Aspirate and remove orogastric tube, ensure optimal CPAP and monitoring |
Give dose of sucrose |
 |
 |
|
Lubricate the back and sides of the I-Gel and the tip of the surfactant giving set |
|
 |
 |
|
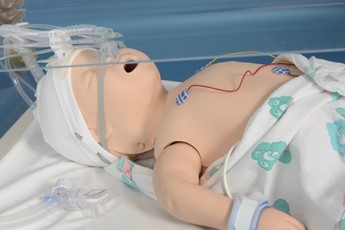
Insert I-Gel and hold in place |
|
 |
 |
|
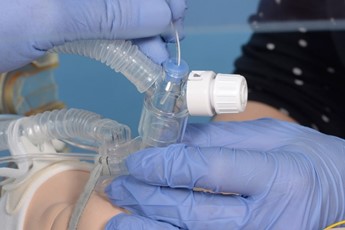
Connect the circuit and ensure pedicap colour change, repositioning the LMA if required. |
|
 |
 |
|
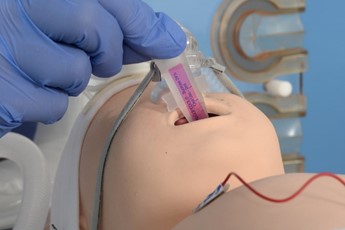
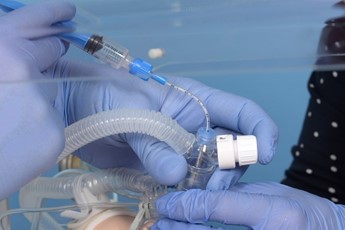
Remove the pedicap and connect the circuit directly to the LMA. |
Introduce the surfactant catheter through the duckbill port. |
 |
 |
|
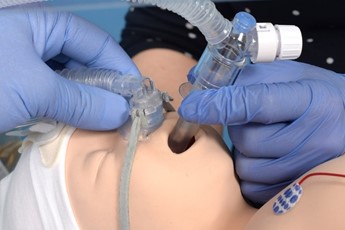
Advance the catheter to 16cm ensuring it has not coiled. A small amount of surfactant can be given to lubricate the lumen of the LMA if needed. |
Give the surfactant slowly over 2-5 mins. In the case of bradycardia, apnoea or desaturation pause and provide IPPV and PEEP as required. |
 |
 |
|
Ensure the nasal CPAP is fitted correctly before removing the LMA. |
Replace and then aspirate the orogastric tube documenting the volume of any surfactant. |
|
Equipment
|
Patient
|
Team Allocate roles
Team leader brief
|
- Nononvasive ventilation with vs without early surfactant to prevent chronic lung disease in preterm infants: a systematic review and meta-analysis. . Isayam T, Chai-Adisaksopha C, McDonald SD.l. : JAMA Pediatrics, 2015, Vol. 169:731.
- Acute lung injury in preterm fetuses and neonates: mechanisms and molecular pathways. . Iliodromiti Z, Zygouris D, Sifakis S, Pappa KI, Tsikouras P, Salakos N, et al.l. : J Matern Fetal Neonatal Med, 2013, Vol. 26.
- Adminstration of Resue Surfactant by laryngeal Mask Airway:lessons from a Pillot Trial. J Attridge, C Stewart, G Stukenborg, J Kattwinkel.l. : American Journal od Perinatology, 2013, Vol. 30.
- Randomized trial of laryngeal mask airway versus endotracheal intubation for surfactant delivery. JMB Pinheiro, Q Santana-Rivas and C Pezzano.l. : Journal of Perinatology, 2016, Vol. 36.
- A randomized controlled trial of the laryngeal mask. Rosilu F. Barbosaa, Ana C. Simões e Silva , Yerkes P. Silva. 4, s.l. : Jornal de Pediatria, 2017, Vol. 93.
- Laryngeal Mask Airway for Surfactant Administration in Neonates: A Randomised Control Trial. Kari D. Roberts, , Roland Brown, Andrea L. Lampland, Tina A. Leone, Kyle D. Rudser,.l. : J Pediatr, 2018.
- Airway rescue and drug delivery in an 800 g neonate with the laryngeal mask airway. J Brimacombe, D Gandini.l. : Paediatric Anaesthsia, 1999.
Last reviewed: 09 October 2020
Next review: 23 October 2023
Author(s): Dr Natalie Smee – West of Scotland Trainee; Dr Joyce O’Shea – Consultant Neonatologist QEH
Co-Author(s): Other Professionals consulted: Jacqueline Rooney – Neonatal Pharmacist Wishaw General Hospital
Approved By: West of Scotland Neonatology Managed Clinical Network

