Transfusion Triggers:
Exchange transfusion is required in infants with severe Haemolytic Disease due to blood group incompatibilities or G6PD deficiency. It is used in these infants to manage severe anaemia at birth Hb <100 g/l, or to treat severe hyperbilirubinaemia which is not controlled by phototherapy and, where appropriate, intravenous immunoglobulin therapy.
The decision to carry out an exchange transfusion will be taken at consultant level but is usually required if the bilirubin exceeds, or is anticipated to exceed, the exchange transfusion threshold on a gestation appropriate phototherapy chart – see separate guideline
Unit Characteristics:
Plasma-reduced ("partially packed") red cells with a haematocrit of 0.5-0.6 are suitable for ET for both hyper-bilirubinaemia and severe anaemia, this should be the only component used for ET. These units should be less than 5 days old. Irradiation is required for VLBW babies and advised for term babies.
Sourcing and cross-matching appropriate units may take some time and requirements must be discussed with blood bank as soon as ET is anticipated. In the case of known, severe haemolysis in-utero, blood will need to be ordered before delivery ideally giving Blood Bank 24 hours of notice. This requires liaison with the obstetric team and Blood Bank in advance of the expected delivery date/time - see separate guidance for the management of the mother with irregular antibodies. If a situation arises unexpectedly whereby an ET is indicated if the mother’s group and antibodies are known, the specialised pack is relatively easy to source from Edinburgh and with the added time for irradiation can be delivered in 3-4 hours. If the mother has rare antibodies then the whole process may take longer, potentially up to eight hours. If the clinical need is more urgent then a discussion between the consultant neonatologist and haematologist should take place to decide whether an alternative suitable unit which is more readily available can be used instead.
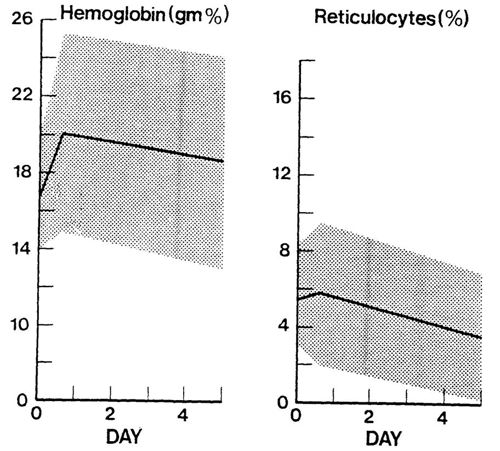
Volume to be exchanged: Twice the circulating blood volume (80ml x 2 = 160ml/kg). Using a double-volume exchange is more effective at removing maternal antibody from the baby’s circulation although even this volume does not remove all the antibody.
Technique: ET may be performed either via a single umbilical venous line or by using a UAC and a UVC combined (blood is withdrawn using the arterial line and replaced via the venous line). Umbilical lines should be placed and position confirmed by x-ray as per WoS policy.
- ET is a sterile procedure.
- Pre ET check FBC, Coagulation, U+E, Ca, Glucose, SBR and blood gas.
- A blood warmer should be used.
- Each aliquot should be approximately 8ml/kg, indeed initial aliquots may be of smaller volume until it is clear the procedure is well tolerated.
- Each aliquot should be removed and replaced over a 5 minutes cycle. Always commence an ET by removing an aliquot of blood. Each aliquot removed and replaced should be timed by the neonatal nurse assisting with ET and recorded on a standard ET recording chart (see appendix).
- The ET should take ~2 hours (longer if poorly tolerated and smaller aliquots are required).
- Blood glucose monitoring is required after every 4 cycles.
Post Transfusion management:
- Repeat the bloods taken prior to the exchange - FBC, Coagulation, U+E, Ca, Glucose, SBR & gas.
- Continue phototherapy
- Continue to monitor serum bilirubin concentrations 4 hourly as a rebound hyperbilirubinaemia may occur.
- While these babies have evidence of haemolysis they should receive folic acid supplementation
Immunoglobulin therapy for Alloimmune Haemolysis
A Cochrane review by Alcock et al. in 2009, indicated that significantly fewer infants treated with intravenous immunoglobulin (IVIG) required Exchange Transfusion (ET), relative risk 0.28 (CI 0.17-0.47). The number needed to treat in order to prevent one ET was 2.7 (CI 2.0-3.8). This result was based on a relatively small number of patients but goes some way to support the use of IVIG in infants with Alloimmune Haemolytic Disease of the Newborn (HDN) in whom the Serum Bilirubin (SBR) continues to rise despite appropriately administered phototherapy – see separate guidelines for the management of jaundice.
Dose: 500mg/kg Intravenous Immunoglobulin given by infusion over 4h. The dose may be repeated once after 48h if required.
Supply: Immunoglobulin is ordered via pharmacy using an Immunoglobulin request form – see appendix of the pharmacy monograph for Immunoglobulin.
Emergency Supplies: A small number of units are kept locally within the neonatal units and in the emergency drug cupboards. See local details below
Local Arrangements
PRM
- 2x vials in the neonatal unit
- Further vials available in A&E and in the emergency drug cupboard in GRI. The latter may be accessed by paging the hospital coordinator via switchboard.
RHC
- Vials are kept in the neonatal unit
- Further supplies are available via pharmacy
RAH
- Vials are kept in the neonatal unit
- Further supplies are available via pharmacy