Contents:
Renal Pelvicalyceal Dilatation (RPD)
|
Antenatal Counselling
|
Consider neonatology counselling for low risk pathway (see flowchart)
Neonatology counselling for high risk pathway (see flowchart) with Nephrology/Urology input.
|
|
Attendance at Delivery/ Admission
|
Not routinely required. NNU admission not routinely required.
|
|
Imaging
|
Low risk pathway - Renal USS 4-6 wks and 6 months.
DMSA indicated if dilatation persists.
High risk pathway – Renal USS 4-14 days + DMSA at 3 months.
|
|
Referral/ Discharge
|
Low risk pathway – Discharge after 6 months if USS findings resolve. Referral to Nephrology/Urology if findings persist
High risk pathway - Referral to Nephrology/Urology
|
|
Prophylaxis
|
High Risk pathway only
|
|
Genetics
|
Referral/ testing not indicated.
|
|
Other Monitoring
|
No routine monitoring for low risk pathway
High risk pathway – may require additional monitoring for severe dilatation
|
Dilatation of the renal pelvis detected on antenatal ultrasound examination has an incidence of between 0.5 and 1% and may be associated with significant renal disease in a small number of babies1. However in the majority of babies the condition is benign and may be classed as isolated renal pelvis dilatation. The challenge, within the population of babies scanned antenatally, is to identify the small number of babies who have significant renal disease requiring later surgery or long term follow-up of renal function without subjecting large numbers of ‘normal’ neonates to unnecessary investigations and antibiotics. Some conditions may require urgent management such as obstruction to urinary flow- posterior urethral valves and pelviureteric junction obstruction (PUJ). Vesicoureteric reflux (VUR) is more common and the benefits of early detection are not clear2. Although there are varied approaches between centres as to the optimum method of antenatal classification, investigation and follow-up, recently there has been a gradual shift upwards in the cut-off value for investigation of renal pelvis dilatation. The following approach reflects the outcomes of recent systematic literature review.
Antenatal Screening for pelvis dilatation
An assessment of the renal system should be mandatory in all pregnancies undergoing a detailed scan and include-
- An assessment of the presence, size and morphology of each kidney
- An exact measurement of the AP pelvic diameter in mm
- An assessment of the presence or absence of calyectasis
- An indication of unilateral or bilateral disease
- An assessment of the bladder wall and distal ureters
- Documentation of any parenchymal abnormality
(e.g. renal cortical thinning, lack of corticomedullary differentiation)
Ultrasound scans should be performed by an experienced sonographer.
Details of renal tract, including the dimensions of each renal pelvis & the presence of any of the features listed above, should be clearly documented in the mother’s notes and in the paediatric communication section. The date of first detection and change over time should be recorded and made available to the paediatric team.
Parents should receive clear advice as to the nature of the problem seen and the course of events following birth. Parent information sheets are available with this guideline (Appendix).
The paediatric team should be informed antenatally if the scan indicates that the baby will fall into the high risk pathway, and there is a need for early investigation (right hand side of the flow chart below). Antenatal counselling of the family by an experienced paediatrician is desirable in these cases.
Cut-off AP measurement for investigation and prophylaxis
Traditionally, a renal pelvic diameter (RPD) cut-off of 5-7mm has been accepted in the second trimester with wide ranging reports of 5mm to 20mm3-7. The most recent review and meta-analysis of the literature, while recognising the limits of mainly observational studies, concludes that isolated AP dilatation less than 12mm has not been associated with significant morbidity and resolved in all cases studied8. Other recent large UK studies have reported little requirement for later intervention other than surveillance for dilatation below 12mm9. At levels above 12 mm the literature is too varied to allow for meaningful analysis however it is clear that an RPD above 15mm confers a higher risk for significant obstruction to urinary flow 1,11,12. However, local audit has shown that infants with normal or low risk RPD but who, in addition, have one or more of the listed risk factors have a higher incidence of problems and must be treated as per the high risk pathway13.
Antibiotic prophylaxis
The benefit of antibiotic prophylaxis to prevent breakthrough infections in this population is unclear. In the mild isolated group of babies, with RPD 10-15mm, there is little evidence that picking up mild vesicoureteric reflux at this stage makes a difference to long term outcome and therefore Trimethoprim prophylaxis is not indicated10. In cases where the baby is at a high risk of urinary obstruction (RPD>15mm) or where there is already evidence of renal damage, antibiotic prophylaxis should be commenced at birth pending results of early imaging. With all babies, appropriate advice must be given to parents on the importance of urine testing if the baby is unwell and prompt treatment of infection if detected. (see appendix)
Timing of follow-up
- Babies in the high risk group with an RPD >15mm or with other risk factors -
calyectasis, parenchymal abnormality, ureterocoele, bladder wall thickening, oligohydramnios, bilateral disease etc require review by a neonatal consultant and rapid USS in the first 2 weeks of life. (N.B. an USS before 72 hours can be misleading and should be avoided if possible. However a small group of children who may require urgent intervention will require the scan in this time period – e.g. Posterior urethral valves). Following confirmation of significant RPD>15mm on USS or other risk factors, as detailed, a DMSA request should be followed by verbal and written referral to nephrology consultant.
- With milder dilatation serial US scans have been shown to be as effective as an MCUG to exclude significant VUR 4,5.
- Babies in the lower risk category should be referred for renal USS at 6-8 weeks age and 6 months age.
- Review of 1st USS will decide the need for further investigation of renal function (DMSA)
Exit from screening
A clear endpoint to follow up is desirable with most patients being categorised as ‘normal’ or ‘requiring further investigation and a renal/urological review’. A third category of baby may be followed up in a general neonatal/paediatric clinic if the DMSA is normal and the dilatation remains between 12-15mm with further investigation and treatment at the discretion of the consultant involved. Please note that in older children a pre- and post-micturition USS should be requested to exclude significant dilatation.
Parent information
Parents should have access to straight forward explanations of the condition at every stage. Information leaflets emphasize the reason for investigation, the reason for prophylactic antibiotics when prescribed and the consideration of urinary tract infection as a cause of illness. (appendix)
Flow Chart For Management of Antenatal Renal Pelvis dilatation
Patients in the highest risk category should already have been discussed with urology and may have an individualised care plan recorded in the notes.

* An USS too early in the first week of life may underestimate the degree of dilatation but may be required for Highest Risk patients if there is suspicion of significant obstruction e.g Urethral valves.
Pelvi-Ureteric Junction (PUJ) Obstruction
|
Antenatal Counselling
|
Consider neonatology counselling for low risk pathway (see RPD flowchart)
Neonatology counselling for high risk pathway (see RPD flowchart) with Nephrology/Urology input.
|
|
Attendance at Delivery/ Admission
|
Not required routinely
|
|
Imaging
|
Renal USS – Urgency indicated as per RPD guideline
|
|
Referral
|
Refer to nephro-urology per indications in RPD guideline.
Ongoing neonatal/ medical follow up as per RPD guideline where nephrology referral not yet indicated.
|
|
Prophylaxis
|
Indicated as per RPD guideline. See table and flowchart).
|
|
Genetics
|
Referral not indicated
|
|
Other Monitoring
|
None required
|
This is the commonest cause of upper urinary tract obstruction. Up to 40% may be bilateral. The majority are primary, due to a dysfunctional segment at the PUJ, which is narrower than normal surrounding ureter. Secondary PUJO occurs due to aberrant lower pole vessels crossing the pelvis which may cause obstruction. Incidence is quoted around 1 in 1000-1500, male:female ratio is 2-3:1. The majority of cases are detected antenatally, otherwise they present with UTI, loin/abdominal pain, haematuria or a palpable flank mass. The renal pelvis and calyces are dilated to a varying degree with no ureteric dilatation. The renal pelvis may be disproportionately dilated compared to the calyces. If the calyces are not dilated it is unlikely to be obstructed. PUJO may be intermittent, depending on urine flow rate. There can be an association with other anomalies, including ectopic kidneys and duplex kidneys (lower pole).
Initial management is the same as that for RPD. Approximately 25% will require surgical intervention with pyeloplasty, which is more likely if RPD >30mm. Indications for surgery include the following:
- Reduced function <40%
- Decreasing function >10%
- RPD >40mm
- Increasing dilatation
- Symptoms such as UTI.
Often surgery will be deferred but in certain circumstances decompression may be required in the neonatal period to preserve renal function with definitive surgery offered once function confirmed. If there are any atypical features they should be discussed urgently. Other triggers for urgent discussion would include: Solitary kidney with PUJ, bilateral disease, significant dilation >30mm, significant progression on sequential scans.
Vesico-Ureteric Junction (VUJ) Obstruction
|
Antenatal Counselling
|
Consider neonatology counselling for low risk pathway (see flowchart)
Neonatology counselling for high risk pathway See flowchart with Nephrology/Urology input.
|
|
Attendance at Delivery/ Admission
|
Not required routinely
|
|
Imaging
|
Renal USS – Urgency indicated as per RPD guideline See table and flowchart.
|
|
Referral
|
Refer to nephrourology per indications in RPD guideline.
Ongoing neonatal/ medical follow up as per RPD guideline where nephrology referral not yet indicated.
|
|
Prophylaxis
|
Indicated as per RPD guideline. See See table and flowchart.
|
|
Genetics
|
Referral not indicated
|
|
Other Monitoring
|
None required
|
Obstruction at the VUJ may cause ureteric dilatation and hydronephrosis, often the degree of renal pelvis and calyceal dilatation is less than with PUJ obstruction. VUJ obstruction can also occur in association with a megaureter, either obstructed, or obstructed and refluxing. Distinction cannot be made on USS alone, although the distal ureter dilatation is often more prominent in VUJ obstruction, with tapering at the VUJ. Many will improve spontaneously, and only approximately 20% require surgical intervention such as a JJ stent or ureteric reimplantation.
Ureterocoele
|
Antenatal Counselling
|
Refer to the neonatal team for discussion with family regards plan for postnatal imaging. Consult with urology where there is associated RPD ≥15mm or bilateral findings.
|
|
Attendance at Delivery/ Admission
|
Paediatric team not routinely required at delivery.
BILATERAL RPD - Admission to NNU. Admission can be deferred for 2-3 hours if clinical condition allows.
UNILATERAL RPD (CONTRALATERAL RENAL PELVIS <10mm) – Admission not required.
|
|
Imaging
|
BILATERAL RPD Request postnatal USS at 24-72 hours of age following urgent referral to urology.
UNILATERAL RPD (CONTRALATERAL RENAL PELVIS <10mm) Postnatal USS day 4-7
|
|
Referral
|
Refer to urology non-urgently when finding of uretercoele confirmed. Immediate referral required in cases with RPD ≥15mm (Unilateral or bilateral or any suggestion of changes in the contralateral kidney).
|
|
Prophylaxis
|
Commence prophylaxis after delivery
|
|
Genetics
|
Referral not indicated
|
|
Other Monitoring
|
BILATERAL RPD ONLY – Monitor urine output, BP, daily U&E.
|
A ureterocoele is a cystic dilatation of the intravesical portion of the ureter that occurs in the bladder wall. They are more commonly associated with a duplex system with a tendency to occur in the ureter draining the upper pole of the kidney. The most common mode of presentation used to be febrile UTI but improved antenatal scanning has increased the number of asymptomatic neonates detected with this condition. In some cases a duplex system raises the possibility of a ureterocoele that is subsequently detected on postnatal imaging. As a result a more conservative approach has been adopted when treating this condition if detected antenatally.
The goal of ureterocoele management includes control of infection, preservation of renal function and protection of normal ipsilateral and contralateral units. In one study of cases detected antenatally rates of associated VUR were up to 51% with 9% of all cases grade III-V. A non-functioning upper pole on DMSA was detected in up to 54% of cases (duplex systems) but this included infants detected ‘symptomatically’ as well as those through antenatal screening. Options for definitive management of ureterocoele depend on the presenting problem but include endoscopic puncture, excision and partial or complete reconstruction of the lower urinary tract and also heminephrectomy of the non-functioning pole.
Cases where there is associated renal pelvis dilatation (RPD) ≥15mm require more urgent assessment. These infants are most likely to have a postnatal management plan in place prior to delivery that should be adhered to. Any changes noted on the contralateral kidney should raise concern that the ureterocele is causing mechanical or functional obstruction to the bladder – this may progress rapidly post natally as the bladder becomes more active and should trigger early discussion and investigation.
Otherwise, infants should commence antibiotic prophylaxis at birth.
Urgent postnatal ultrasound should be requested on day 4-7.
If the postnatal scan is normal, antibiotics can be stopped and no further follow up is required.
When the diagnosis is confirmed infants should have an MCUG and DMSA. Refer to urology to ascertain appropriate timing for MCUG & DMSA and consideration of operative management.
Megaureter
Unilateral Megaureter
|
Antenatal Counselling
|
Consider referring family to Neonatal team for discussion regards postnatal management.
|
|
Attendance at Delivery/ Admission
|
Not required routinely.
|
|
Imaging
|
Renal USS – urgent in 4-7 days.
MCUG / MAG3 may be required – discuss with urology once USS results known
|
|
Referral
|
Non-urgent referral to urology with ultrasound findings.
|
|
Prophylaxis
|
Commence prophylactic trimethoprim before discharge.
|
|
Genetics
|
Referral not indicated
|
|
Other Monitoring
|
None required
|
Bilateral Megaureter OR Unilateral Megaureter with absent or dysplastic contralateral kidney.
|
Antenatal Counselling
|
Refer family to Neonatal team for discussion regards postnatal management. NB – In boys this appearance may suggest posterior urethral valves
|
|
Attendance at Delivery/ Admission
|
Attendance at delivery not required routinely. Admission to NNU will be required for accurate assessment of renal function and urine output. Note male infants will require immediate urinary catheterisation prior to urology assessment.
|
|
Imaging
|
Urgent renal USS – 24-48 hours.
|
|
Referral
|
Urgent referral to nephrology & urology within 24-48 hours.
|
|
Prophylaxis
|
Commence antibiotic prophylaxis after delivery.
|
|
Genetics
|
Referral not indicated
|
|
Other Monitoring
|
Daily assessment of BP, U&E’s, urine output and fluid balance pending USS and discuss with urology.
|
This is an abnormally wide ureter; classed as obstructed, refluxing, obstructed and refluxing or non-refluxing/non-obstructed. Secondary megaureter is due to distal obstruction i.e. urethral obstruction, bladder outlet obstruction etc. Primary megaureter occurs with cases of obstructed and non-obstructed megaureter, where secondary causes have been excluded.
- Non obstructed: The majority of cases are non obstructed with no evidence of VUR. The exact aetiology is unclear.
- Obstructed: Functional obstruction occurs due to an aperistaltic section of the distal ureter causing an inability to transport urine.
There is an increased incidence in boys and megaureter occurs more commonly in the left ureter. 25% of cases are bilateral. If unilateral approximately 10-15% will have an absent or dysplastic contralateral kidney. Most cases are diagnosed antenatally.
Management will vary similarly to the RPD guideline (page 3) where cases with bilateral involvement require more urgent assessment.
NB Secondary megaureters may require urgent treatment of the primary cause. In male infants these findings may represent a presentation of posterior urethral valves which require urgent intervention. The antenatal findings cannot differentiate this group so in male infants urgent discussion is mandatory.
Posterior Urethral Valves
|
Antenatal Counselling
|
If severe (>15mm) bilateral RPD or oligohydramnios, referral to Neonatal and Urology teams is necessary and a postnatal management plan must be documented.
|
|
Attendance at Delivery/ Admission
|
Attendance not required routinely unless there is severe oligohydramnios. Followed in all cases by admission to NNU – this may be deferred for 2-3 hours if the infant’s clinical condition allows. On admission all should have IV access established; baseline bloods (accepting that this will reflect maternal renal function – but important as a first point in the trend) and urinary catheterisation. Close monitoring for electrolyte changes associated with post obstructive diuresis.
|
|
Imaging
|
Urgent renal USS within 24-48 hours of delivery unless otherwise specified by a management plan agreed up on antenatally. Early MCUG will be required to confirm the diagnosis – discuss with urology.
|
|
Referral
|
Urgent referral to Urology and Nephrology if suspicion of this diagnosis or if identified at postnatal USS. May already have a pathway agreed up on in antenatal period
|
|
Prophylaxis
|
Commence antibiotic prophylaxis following USS confirmation or sooner if pre-agreed.
|
|
Genetics
|
Referral not indicated
|
|
Other Monitoring
|
Daily U&E’s, blood pressure, measurement of urine output and fluid balance (majority will need fluid and electrolyte replacement in the early phase). If the condition is suspected (or confirmed) establishment of bladder drainage is mandatory while other neonatal care continues and further investigations are arranged. Post catheterisation one must anticipate a post obstructive diuresis and fluid shifts which require careful fluid balance management
|
Posterior urethral valves are a congenital malformation of the posterior urethra, only seen in male infants. Valves affect the developing urinary tract from an early stage, and lead to bladder outlet obstruction which can cause VUR, hydronephrosis, and calyceal rupture. Valves can lead to pulmonary hypoplasia if oligohydramnios has developed secondary to urinary obstruction. The degree of renal impairment depends on the degree of renal dysplasia, either integral to the condition, or secondary to obstruction. A significant proportion of these infants will progress to end stage renal failure before adulthood despite aggressive and appropriate therapy. Posterior urethral valves is a chronic condition requiring life long monitoring and treatments. Antenatal treatments have been proposed for valves boys but at present the data does not support improvement in renal outcomes sufficient to justify routine intervention outside trials.
Posterior urethral valves are exclusive to male infants. Rarely anterior urethral valves affect females. Estimated incidence 1 in 5000 –12000. 40-60% are seen on antenatal USS with oligohydramnios, bilateral hydronephrosis and hydroureters, bladder wall thickening and posterior urethral dilatation. Occasionally PUV’s can be missed without a third trimester scan. 10% of boys with posterior urethral valves have unilateral hydronephrosis, as such the diagnosis must be considered even with unilateral changes.
Oligohydramnios can cause postural defects such as talipes, dislocated hip, receding jaw ie Potter like syndrome. Severe oligohydramnios and resulting pulmonary hypoplasia can be fatal in the neonatal period.
Renal function at birth can vary from normal to severely impaired. Tubular damage leads to sodium and bicarbonate wasting, with secondary hyperkalaemia. Following surgical treatment of valves and relief of obstruction, creatinine may normalise, or remain significantly elevated, dependent on the degree of renal dysplasia.
Cases with significant antenatal bilateral hydronephrosis and/or oligohydramnios should be discussed antenatally with urology and a postnatal plan documented.
If there is bilateral hydronephrosis and suspicion of PUV’s, arrange an urgent USS within the first 48 hours. Discuss with the neonatal consultant and urology. Urinary catherisation should be performed in all cases to relieve obstruction pending further imaging and investigation.
MCUG is the gold standard imaging for diagnosis. It will show posterior urethral dilatation and thickened bladder wall with an abnormal trabeculated contour, unilateral or bilateral VUR may be present. In cases where MCU is non diagnostic or were the index of suspicion is high it may still be appropriate to consider cystoscopy. It is possible for cases of PUV’s not to be detected on MCUG especially where there is high grade reflux.
While awaiting surgery following catheterisation there needs to be careful attention to fluid and electrolyte balance as post obstructive diuresis occurs and these babies will often need additional sodium and bicarbonate. Joint management with Nephrology is essential to optimise the renal recovery.
Treatment is surgical. The valves are ablated cystoscopically. If valve ablation is not possible urinary diversion with vesicostomy or bilateral ureterostomies may be needed with later valve surgery and subsequent recontsruction.
Long term follow up will be shared by the Nephrology and Urology teams. Ongoing USS monitoring for renal tract dilatation will be required. Repeat cystoscope or MCUG at approximately three months is needed to ensure there are no residual valve leaflets.
DMSA at approximately three months after relief of obstruction and resolution of infection is needed to assess the degree of renal dysplasia, along with monitoring of renal function. However, outcomes should remain guarded and life long monitoring will be required. Normal renal function at 2 years of life is a good prognostic sign but even in this group CKD in later life is expected.
Familial Vesicoureteric Reflux (VUR) – Screening siblings of affected children.
Screening asymptomatic siblings of affected children.
|
Antenatal Counselling
|
Not routinely required.
|
|
Attendance at Delivery/ Admission
|
Not routinely required. NNU admission not required.
|
|
Imaging
|
Renal USS 4-6 weeks – Follow RPD guideline with findings.
|
|
Referral/ Discharge
|
Pending Postnatal USS findings. Indicated as per RPD guideline. See See table and flowchart.
|
|
Prophylaxis
|
Only if renal pelvis dilatation detected antenatally. Indicated as per RPD guideline. See table and flowchart.
|
|
Genetics
|
Referral/ testing not indicated.
|
|
Other Monitoring
|
None required.
|
Vesicoureteric reflux in children has a prevalence of 1-2%. In the majority of cases the degree of reflux is mild and such cases commonly self resolve later in childhood. However there is a smaller group with more severe VUR who are at increased risk of complications such as pyelonephritis and renal scarring.
The prevalence of VUR is known to be increased within families. Recent studies show an increase in prevalence to 27.4% amongst children with an affected sibling or 35.7% amongst children with an affected parent. The prevalence is higher if screening is performed at < 2y of age as the condition naturally improves with age. High grade reflux (Grade III – V) is identified in a minority (9.8% of those screened) confirming that severe reflux, within families, is less common. Renal cortical abnormalities were identified in 14.5% of screened individuals without prior UTI. Overall (symptomatic and asymptomatic) the prevalence amongst family members screened was 22.5%.
Screening 1st degree relatives of those with VUR may have the potential to facilitate earlier identification and if necessary earlier intervention for individuals with VUR. However, due to a lack of prospective randomised studies it is unclear whether such screening will be effective in preventing UTI’s and renal scarring in the long term.
For the purposes of this guideline the following recommendations are made
- Screening should be offered where there is a family history, in a first degree relative, of high grade reflux (Grade III-V) or reflux associated with urinary tract infection or renal scarring. Screening by USS should be offered as a default if the grade of reflux in the relative is unknown.
- Screening should be with Renal ultrasonography at 4-6 weeks
- Additional investigations are only warranted if there are abnormalities on the renal ultrasound. Refer to relevant sections of this guideline
- If the infant is the index case then screening may be recommended for older siblings who are not yet toilet trained. (there is no additional benefit to screening older children who are toilet trained)
Duplication of the Ureters (Duplex Collecting System/ Duplex Kidney(s))
|
Antenatal Counselling
|
Consider referral to neonatal team for discussion with family regards plan for postnatal imaging.
|
|
Attendance at Delivery/ Admission
|
Not routinely required.
|
|
Imaging
|
Unilateral involvement – Renal USS 4-6 weeks
Bilateral involvement – Renal USS 4-7 days
Associated RPD – follow RPD guideline. See table and flowchart.
|
|
Referral/ Discharge
|
Referral to nephrology team if:
1. Evidence of RPD on postnatal USS – follow RPD guideline. See table and flowchart.
Ongoing Neonatal/ medical follow up:
1. If renal USS does NOT demonstrate RPD or any other abnormality other than a unilateral or bilateral duplex system - DISCHARGE
|
|
Prophylaxis
|
Not required unless renal tract dilatation present on antenatal or postnatal USS. See RPD Guideline table and flowchart.
|
|
Genetics
|
Referral not indicated.
|
|
Other Monitoring
|
None required.
|
A duplex or duplicated system is defined as a kidney with two pelvicalyceal systems with or without duplication of the ureter. In incomplete duplication, both ureters join before entering the bladder with a bifid pelvis in the mildest form. In cases of complete duplication, two ureters enter the bladder separately. Incomplete duplication is more common than complete duplication in a ratio of 3:1, with a reported incidence of all forms of 1:125 individuals. Rarely, there may be bilateral duplex systems.
Less often, there are associated renal abnormalities include vesicoureteric reflux, PUJ obstruction (much less likely in complete duplication) and in complete duplication alone, ectopic ureterocoele or ectopic ureteral insertion.
Infants with an antenatal diagnosis should receive a postnatal ultrasound urgently for bilateral duplex kidneys (4-7 days) and routinely for unilateral duplex systems (4-6 weeks). Cases should be considered more urgently where there is renal pelvis dilatation found antenatally. Prophylactic antibiotics are indicated as per the RPD guideline.
If, following postnatal ultrasound, there are other findings, such as pelvicalyceal or ureteric dilatation follow the RPD guideline regards further imaging and referral. See table and flowchart.



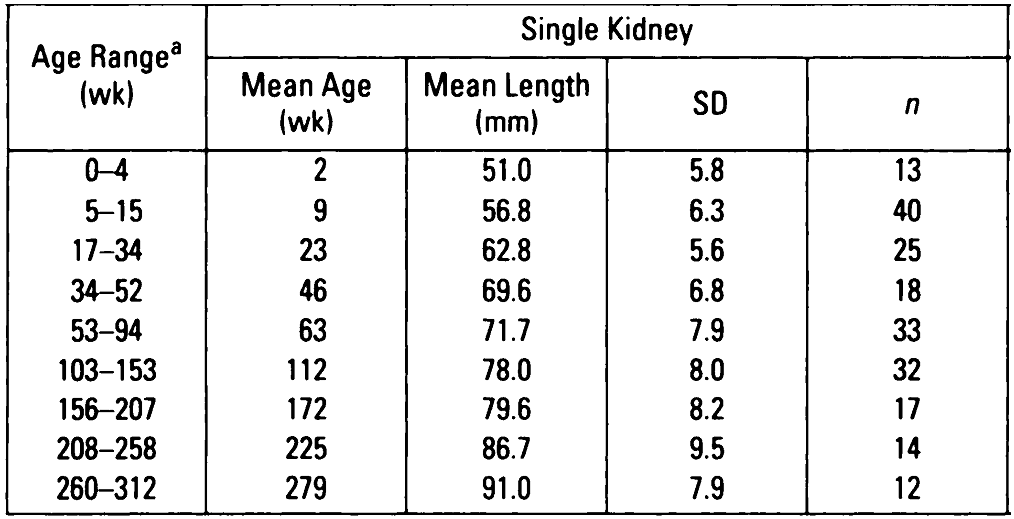 Rottenberg GT; De Bruyn R; Gordon I. Sonographic standards for a single functioning kidney in children.
Rottenberg GT; De Bruyn R; Gordon I. Sonographic standards for a single functioning kidney in children.