(1) van Rooij LGM, Hellström-Westas L, de Vries LS. Treatment of neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):209-215.
(2) Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol 2012 Feb;46(2):111-115.
(3) Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: Saunders Elsevier; 2008.
(4) Slaughter LA, Patel AD, Slaughter JL. Pharmacological Treatment of Neonatal Seizures: A Systematic Review. Journal of Child Neurology 2013 March 01;28(3):351-364.
(5) Pisani, Francesco C, Caterina F, Carlo S, Lisa. Neonatal status epilepticus vs recurrent neonatal seizures: Clinical findings and outcome. Neurology 2007 December 4;69(23):2177-2185.
(6) Pressler RM, Mangum B. Newly emerging therapies for neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):216-223.
(7) Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: A population-based study. Neurology 2007 November 6;69(19):1816-1822.
(8) Glass HC WE. Controversies in Neonatal Seizure Management. Journal of Child Neurology May 2009;24(5):591-599.
(9) Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):185-191.
(10) Pressler RM. Chapter 6: Neonatal Seizures. 2015.
(11) Bassan H, Bental Y, Shany E, Berger I, Froom P, Levi L, et al. Neonatal Seizures: Dilemmas in Workup and Management. Pediatr Neurol 2008 6;38(6):415-421.
(12) Uria-Avellanal C, Marlow N, Rennie JM. Outcome following neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):224-232.
(13) Evans D LM. Neonatal Seizures. Archives of Disease in Childhood. Fetal and Neonatal Edition. 1998;78(1):F70-75.
(14) Booth D, Evans David J. Anticonvulsants for neonates with seizures. Cochrane Database of Systematic Reviews. 2004(3).
(15) Granelli SL, McGrath JM. Neonatal seizures: diagnosis, pharmacologic interventions, and outcomes. J Perinat Neonatal Nurs 2004 Jul-Sep;18(3):275-287.
(16) Mizrahi EM KP. Diagnosis and Management of Neonatal Seizures. Philadelphia: Lippincott-Rave; 1998.
(17) Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):175-184.
(18) Levene M. Recognition and management of neonatal seizures. Paediatrics and Child Health 2008 4;18(4):178-182.
(19) Sivaswamy L. Approach to neonatal seizures. Clin Pediatr 2012 May;51(5):415-425.
(20) Zupanc ML. Neonatal Seizures. Pediatric Clinics of North America 2004;51:961-978.
(21) Glass,H.C, Glidden D, Jeremy RJ, Barkovich AJ, Ferreira DM, MIller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic ischemic brain injury. The Journal of Pediatrics 2009;155(3):318-323.
(22) Lynch J. Epidemiology and Classification of Perinatal Stroke. Seminars in Fetal and Neonatal Medicine 2009;14:245-249.
(23) Silverstein FS JF. Neonatal Seizures. Ann Neurol 2007;62:112-120.
(24) Laine K, Heikkinen T, Ekblad U, Kero P. Effects of Exposure to Selective Serotonin Reuptake Inhibitors During Pregnancy on Serotonergic Symptoms in Newborns and Cord Blood Monoamine and Prolactin Concentrations. Arch Gen Psychiatry 2003;60(7):720-726.
(25) Haddad PM, Pal BR, Clarke P, Wieck A, Sridhiran S. Neonatal symptoms following maternal paroxetine treatment: Serotonin toxicity or paroxetine discontinuation syndrome? Journal of Psychopharmacology 2005 September 01;19(5):554-557.
(26) Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. The Lancet 2005 2/5–11;365(9458):482-487.
(27) Sheth RD, hobbs GR, Mullett M. Neonatal Seizures: incidence, onset and aetiology by gestational age. J Perinatol 1999;19:40-43.
(28) Dehan M, Gabilan JC, Navelet Y, D'Allest Am. Fifth day fits. Archives of Disease in Childhood 1982;57:400-401.
(29) Rennie JM, Boylan GB. Neonatal seizures and their treatment. Curr Opin Neurol 2003 Apr;16(2):177-181.
(30) Helen Cross J. Differential diagnosis of epileptic seizures in infancy including the neonatal period. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):192-195.
(31) Hallberg B, Blennow M. Investigations for neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):196-201.
(32) Jensen FE. Neonatal Seizures: An Update on Mechanisms and Management. Clin Perinatol 2009;36(4):881.
(33) Boylan GB, Stevenson NJ, Vanhatalo S. Monitoring neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):202-208.
(34) Shellhaas RA, Barks AK. Impact of amplitude-integrated electroencephalograms on clinical care for neonates with seizures. Pediatr Neurol 2012 Jan;46(1):32-35.
(35) Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med 1999 Aug 12;341(7):485-489.
(36) Castro Conde JR, Hernandez Borges AA, Domenech Martinez E, Gonzalez Campo C, Perera Soler R. Midazolam in neonatal seizures with no response to phenobarbital. Neurology 2005 Mar 8;64(5):876-879.
(37) Sirsi D et al. Successful Management of Refractory Neonatal Seizures with Midazolam. Journal of Child Neurology 2008;23(6):706-709.
(38) Sheth RD, Buckley DJ, Gutierrez AR et al. Midazolam in the treatment of refractory neonatal seizures. Clinical Neuropharmacology 1996;19:165-1.
(39) van den Broek MP, Huitema AD, van Hasselt JG, Groenendaal F, Toet MC, Egberts TC, et al. Lidocaine (lignocaine) dosing regimen based upon a population pharmacokinetic model for preterm and term neonates with seizures. Clin Pharmacokinet 2011 Jul;50(7):461-469.
(40) Lundqvist M, Ågren J, Hellström-Westas L, Flink R, Wickström R. Efficacy and safety of lidocaine for treatment of neonatal seizures. Acta Paediatrica 2013;102(9):863-867.
(41) Boylan GB, Rennie JM, Pressler RM, et al. Phenobarbitone, neonatal seizures and video-EEG. Archives of Disease in Childhood 2002;86:165-170.
(42) van Rooij LG, van den Broek MP, Rademaker CM, de Vries LS. Clinical management of seizures in newborns : diagnosis and treatment. Paediatr Drugs 2013 Feb;15(1):9-18.
(43) McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurologu 2000;55:506-513.
(44) West CR, Harding JE, Williams CE. Cot-side electroencephaliography for outcome prediction in preterm infants: observational study. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2011;96:F108-F112.
(45) Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, et al. The Current Etiologic Profile and Neurodevelopmental Outcome of Seizures in Term Newborn Infants. Pediatrics 2006 April 01;117(4):1270-1280.
(46) van Rooij LGM, de Vries LS, Handryastuti S, Hawani D, Groenendaal F, van Huffelen AC, et al. Neurodevelopmental Outcome in Term Infants With Status Epilepticus Detected With Amplitude-Integrated Electroencephalography. Pediatrics 2007 August 01;120(2):e354-e363.
(47) Pressler RM, Boylan GB, Morton M et al. Early serial EEG in hypoxic ischaemic encephalopathy. Clinical Neurophysiology 2001;112:31-37.
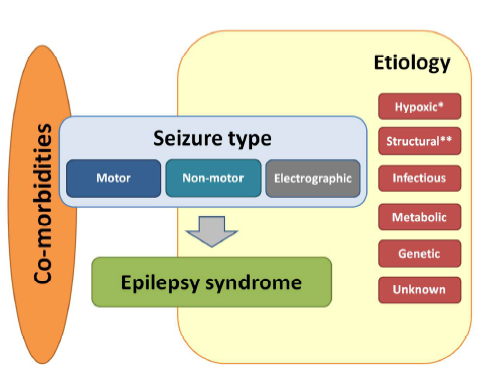
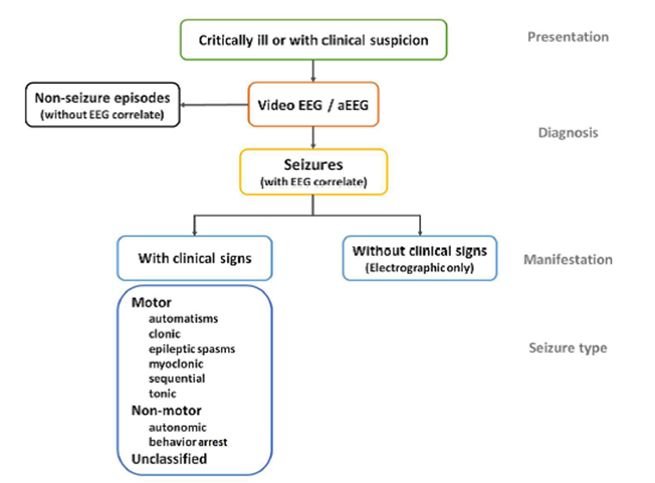
(48) Pressler RM, Cilio MR, Mizrahi EM, Moshes SL, Nunes ML, Plouin P, Vanhatalos S, Yozawitz E, Zuberi S. The ILAE Classification of Seizures and the Epilepsies: Modification for Seizures in the Neonate. Proposal from the ILAE Task Force on Neontal Seizures. ILAE website, 2018.
(49) Gonsales MC, Montenegro MA, Soler VC, Coan AC, Guerreiro MM, Lopes-Cendes I. Recent developments in the genetics of childhood epileptic encephalopathies: impact in clinical practice. B Arq Neuropsiquiarti 2015:1-13
(50) Cornet MC, Sands TT, Cilio MR. Neonatal epilepsies: Clinical management. Seminars in Fetal & Neonatal Medicine 2018;23:204-212
(51) Vilan A, Ribeiro JM, Striano P, Weckhuysen S, Weeke LC, Brilstra E, de Vries LS, Cilio MR. A distinctive amplitude-integrated electroencephalography pattern in newborns with neonatal epilepsy associated with KCNQ2 mutations. Neonatology 2017;112:387-393
(52) Mruk AL, Garlitz KL, Leung NR. Levetiracetam in neonatal siezures: a review. Journal of Paediatric Pharmacology 2015;20(2):76-89
(53) Ahmad KAA, Desai JJ, Bennett MM, Ahhad SF, Ng Y-T, Clark RH, Tolia VN. Changing anti-epileptic drug use for seizures in US neonatal intensive care units from 2005 to 2014. Journal of Perinatology 2017;37:296-300
(54) Han JY, Moon CJ, Youn YA, Sung IK, Lee IG. Efficacy of levetiracetam for neonatal seizures in preterm infants. BMC Pediatrics 2018;18:131
(55) Sharpe C, Reiner GE, Davis SL, et al. Levetiracetam Versus Phenobarbital for Neonatal Seizures: A Randomized Controlled Trial. Pediatrics. 2020;145(6):e20193182).
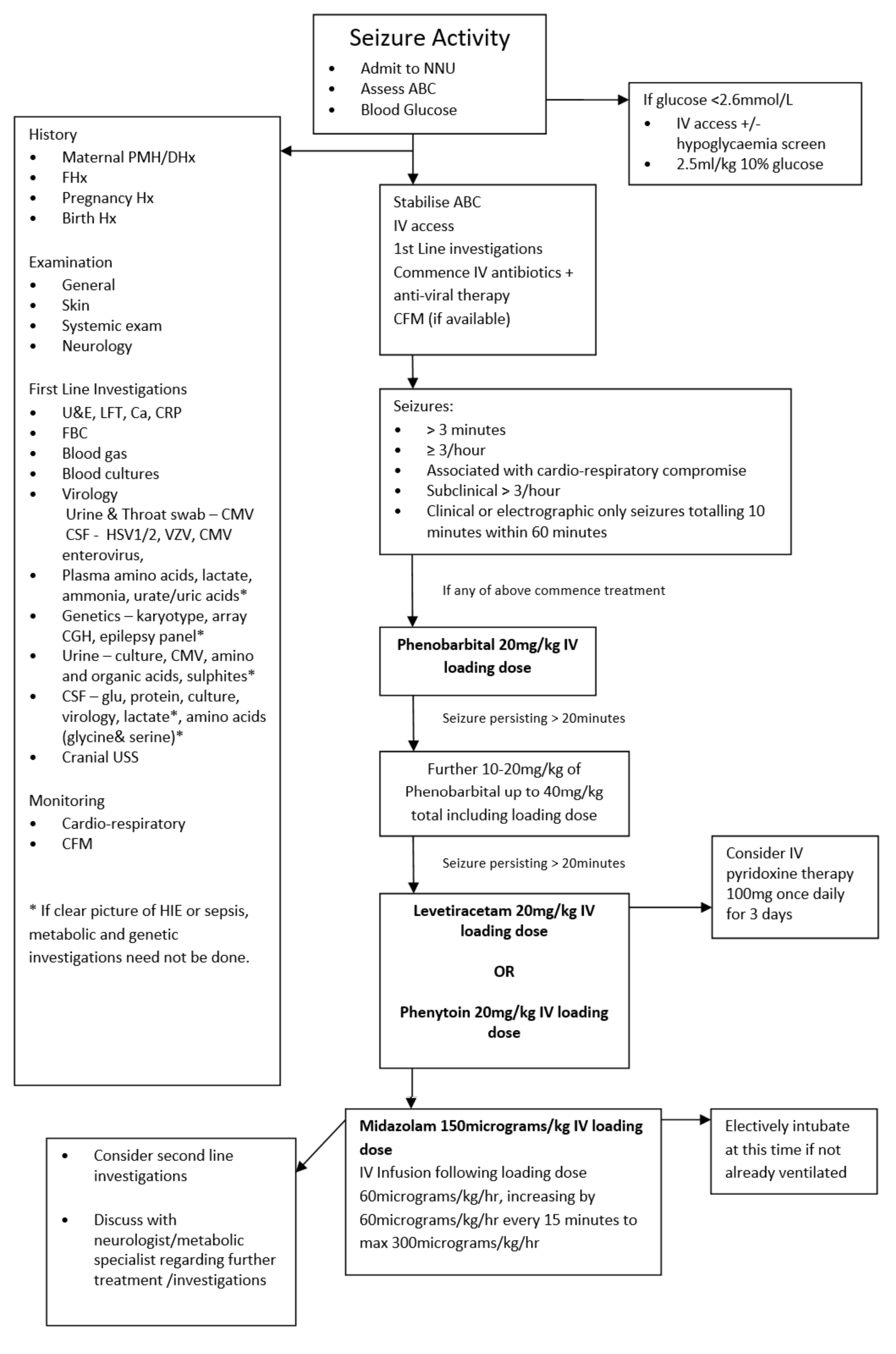




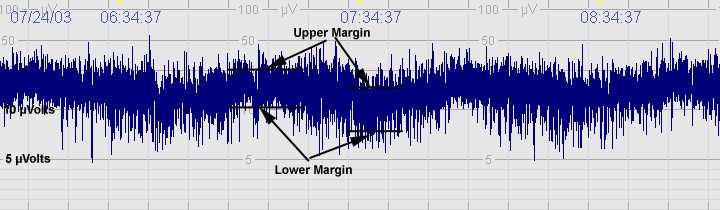
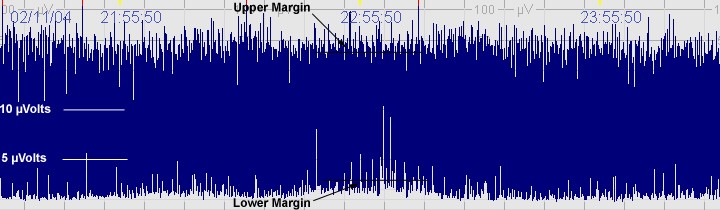
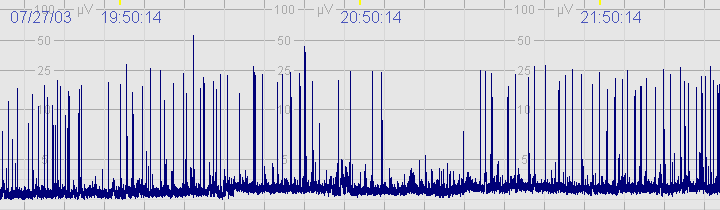 The above CFM trace shows a severely abnormal tracing with no sleep wake cycling seen. The upper limit is <10μvolts and there is very little variability.
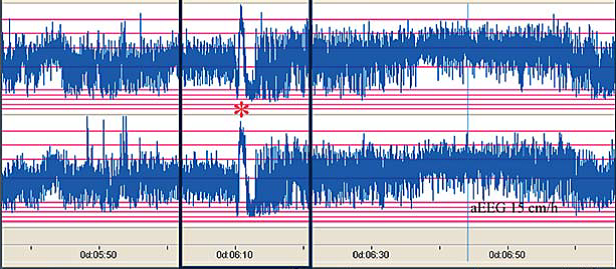
The above CFM trace shows a severely abnormal tracing with no sleep wake cycling seen. The upper limit is <10μvolts and there is very little variability.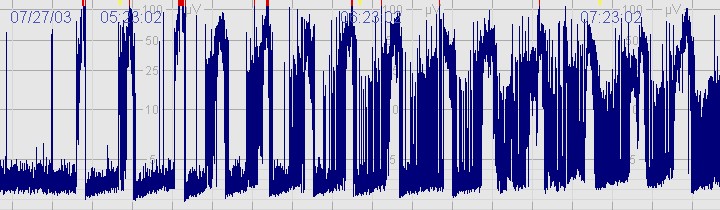 The above CFM tracing shows frequent seizures.
The above CFM tracing shows frequent seizures.