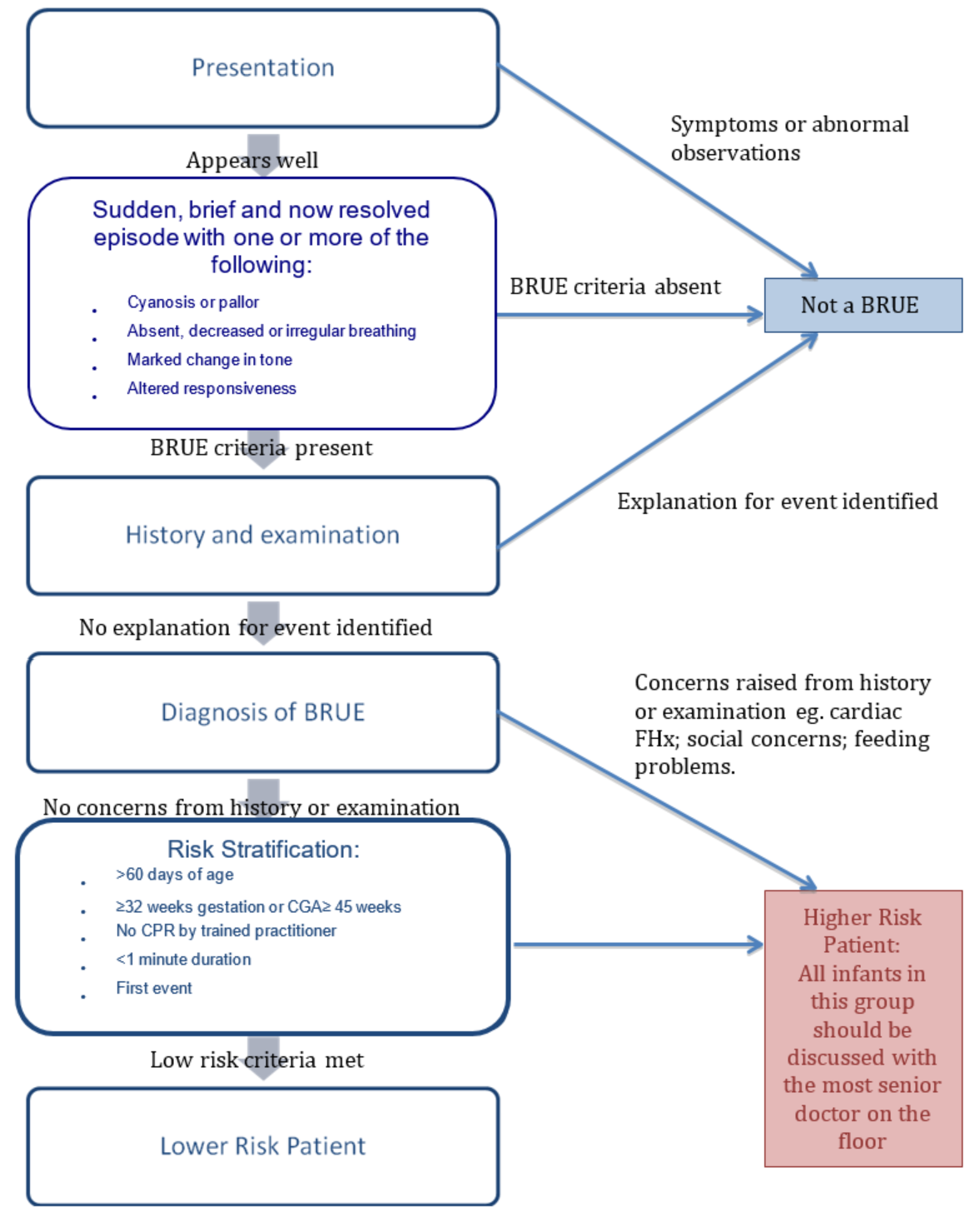
By definition if patient requiring ongoing treatment, episode is NOT a BRUE. Lower risk patients do not routinely need admitted for cardio-respiratory monitoring.
Lower risk: Discharge home only if:
- Low clinical suspicion of serious underlying disorder.
- Parents reassured and happy to care for child at home. If not for discussion with medical team for period of inpatient observation.
Provide parents carers with education / advice on BRUE, including worsening statement for representation.
Lower risk does not mean no risk. Lower risk patients could be considered for a period of observation within the ED department if required.
Higher risk: Following discussion with ED Consultant or OOH senior paediatric registrar, consider admission for observation, cardio-respiratory monitoring and further investigations as guided by presentation.
Involve and refer to relevant specialties as appropriate if underlying cause identified:
- Paediatric medicine
- Paediatric general surgery or neurosurgery - Other (e.g. medical or surgical subspecialties)
- Social work/child protection unit if appropriate.



 Source: American Academy of Pediatrics;
Source: American Academy of Pediatrics;