|
All NAC prescriptions should be on the ‘Acetylcysteine Prescribing and Administration Chart for 12-hr shortened N-acetylcysteine dosing schedule (SNAP protocol) Please ensure you have selected to print on both sides before printing out the chart and ensure it is kept in the patient’s nursing notes |
RHCG Paracetamol overdose guidance - 12-hr shortened N-acetylcysteine dosing schedule (SNAP protocol)

What's new / Latest updates
12/06/2023 This guidance replaces any previous RHCG paracetamol overdose guidance or calculators.
For >6 years of age only
Who is the guidance for:
- Children aged 6 years and over
If under 6 then refer to toxbase for guidance AND discuss with ED / acute paediatric team senior. - For use in NHS Greater Glasgow and Clyde (NHS GG&C) only.
Does the patient require N-acetylcysteine (NAC)?:
For patients aged 6 years and over - If the patient has ingested less than 75 mg/kg of paracetamol, it is unlikely that serious toxicity will occur. For all other cases see specific assessment and treatment flowcharts below.
- Intentional paracetamol overdose in a single time period (ingested total overdose in less than a 1 hour time period):
- Staggered intentional paracetamol overdose (Ingested total overdose in more than 1 hour time period in the context of self harm. Includes patients where time of ingestion is unknown).
- Serious toxicity may occur in patients ingesting >150mg/kg in any 24 hour period, rarely toxicity may occur in patients ingesting 75 - 150 mg/kg in any 24 hour period
- Serious toxicity may occur in patients ingesting >150mg/kg in any 24 hour period, rarely toxicity may occur in patients ingesting 75 - 150 mg/kg in any 24 hour period
- Therapeutic excess paracetamol overdose (ingested total overdose in more than 1 hour time period with NO self harm intent).
|
All NAC prescriptions should be on the ‘Acetylcysteine Prescribing and Administration Chart for 12-hr shortened N-acetylcysteine dosing schedule (SNAP protocol). Please ensure you have selected to print on both sides before printing out the chart and ensure it is kept in the patient’s nursing notes |
RHC Glasgow Emergency Department does not currently use HEPMA for electronic prescribing. In the event of N-Acetylcysteine being started for a patient in ED the following process should be followed.
|
All NAC prescriptions should be on the ‘Acetylcysteine Prescribing and Administration Chart for 12-hr shortened N-acetylcysteine dosing schedule (SNAP protocol)’. This is printed using the links above. This paper document, displaying the rate of infusion for each individual bag, should remain with the patients’ nursing notes until infusion is completed at which time it should be filed in the medical notes. *It should be printed by selecting print BOTH SIDES on the printer options* |
In addition to the above prescription form a paper drug Kardex should be started for the patient. In the once only medication section the N-Acetylcysteine should be Prescribed (including date and prescriber signed/printed) in the ‘ONCE ONLY AND PREMEDICATION DRUG’ section as per below example:
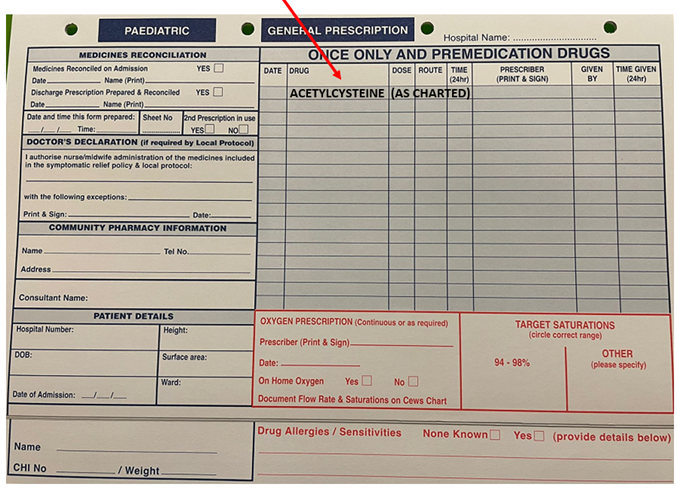
If prescribing NAC in ED for transfer to ward select Inpatient from the main menu, you can then search for the patient with their CHI number.
Ensure patient’s weight & allergy status are entered and are up to date.
Select inpatient from the menu tab at the top of the grey box, once you have done this you can then select the Add Drug tab.
HEPMA requires you to search for the drug by formulary name, it will not accept NAC or N-Acetylcysteine, just enter Acetylcysteine.
You should then select;
- Acetylcysteine Injection for Infusion AS CHARTED Intravenous Continuous Infusion
Once selected a pop-up will appear asking for dose, number of ampules and frequency, before entering this information you will need to change the type of order. This is done by selecting a tab at the top of the pop-up which gives you the choice of regular, PRN or a STAT dose.
Please select STAT as each bag will need to be prescribed individually and it deletes frequency so there is no risk of continued prescriptions.
Prescribing STAT doses also prompts prescribers to check the protocol regularly.
Dose & number of ampules should be the same and each weight bracket on the NAC Protocol will inform you of the number of ampules required.
|
All NAC prescriptions should be on the ‘Acetylcysteine Prescribing and Administration Chart for 12-hr shortened N-acetylcysteine dosing schedule (SNAP protocol)’. This should be printed, ensuring "print both sides" is selected on the printer options, displaying the rate of infusion for each individual bag, and it should remain with the patient’s nursing notes. |
Acetylcysteine Antidote Adverse Effects – Features & Management [PDF for printing]
Management of side effects
- N-acetylcysteine may cause anaphylactoid reactions in 2% of cases with this protocol.
- Flushing, pruritus, rash, hypotension, angioedema, bronchospasm and vomiting are most common.
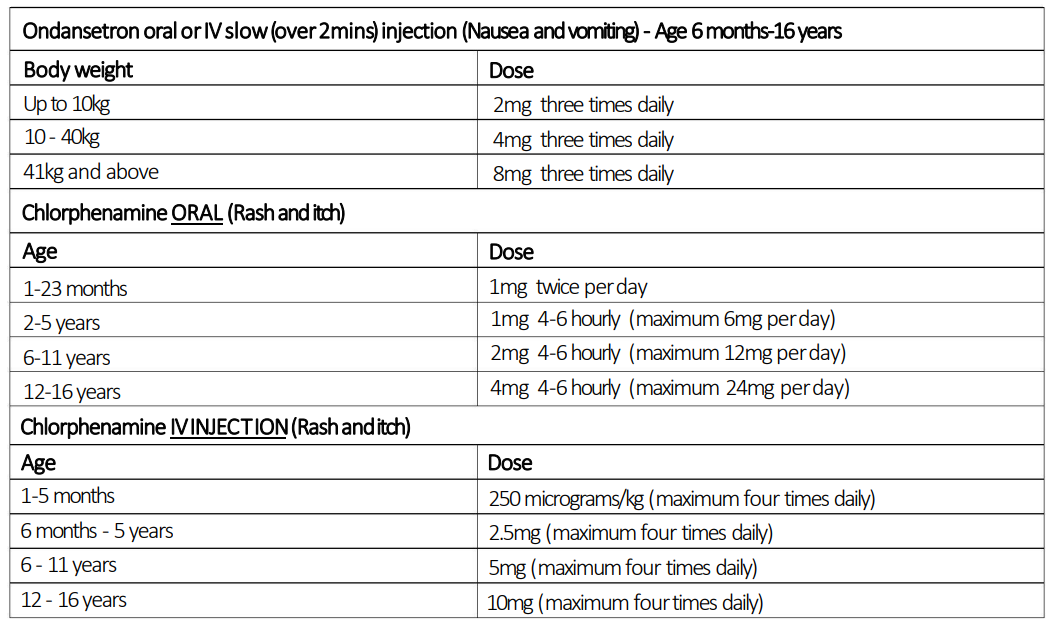
- Reactions can be managed by stopping the infusion. Consider chlorphenamine for flushing/itch, nebulised salbutamol if there is bronchospasm and ondansetron if there are GI side effects.
- Restart the infusion once the reaction has resolved at half the rate to completion of infusion.
- Previous reaction is NOT a contra-indication to N-acetylcysteine and cases should receive treatment if indicated. Reactions are now considerably less common with the 12-hour SNAP protocol compared to standard regimes
Both paracetamol and acetylcysteine treatment may cause an increase in INR in the absence of liver injury.
Patients who do not meet any of the criteria for continuation of acetylcysteine treatment but have an increase in INR of 0.4 or less (e.g. 1.1 to 1.5) and have a normal ALT can be considered for discharge.
For patients who have an increase in INR of 0.5 or more (e.g. 1.1 to 1.6) in the absence of an ALT rise, stop acetylcysteine treatment and recheck INR and ALT after 4 - 6 hours.
After this 4 - 6 hour period without acetylcysteine, the patient can be considered for discharge if the blood tests meet the following criteria:
- INR is unchanged or falling
AND
- ALT is less than two times the upper limit of normal
If the criteria above are not met - restart acetylcysteine at the dose and infusion rate used in the last treatment bag.
Question 1:
Why does TOXBASE advice for ‘Paracetamol overdose ingested over a period of one hour or less - presenting less than 8 hours after acute ingestion - Adults and children 6 years of age and over’ say:
“There is normally no indication to start acetylcysteine without a paracetamol blood concentration provided the result can be obtained and acted upon within 8 hours of ingestion. If there is going to be delay beyond 8 hours after the overdose in obtaining the paracetamol concentration, treatment should be started if 150 mg/kg or more paracetamol has been ingested”.
But the RHC SNAP guideline has the threshold dose for the same group of patients at >75mg/kg?
Answer 1:
The threshold of 75mg/kg used in RHC guideline rather than the 150mg/kg referenced on TOXBASE was decided to ensure no-one with toxic level ingestion has an unnecessary delay in treatment whilst waiting blood results - something that could result in greater injury.
As per Toxbase - TREATMENT MUST BE STARTED WITHIN 8 HOURS IF MAXIMUM PROTECTION IS TO BE OBTAINED.
This is offset by a potential small number of patients who start on acetylcysteine and when results returned it transpires that they are below treatment threshold - if this is identified then the patient can have the treatment stopped.
The local implementation committee felt this option to be the lesser risk and also avoids potential confusion for the user where threshold figures change within the same flowchart from 75mg/kg to 150mg/kg.
Question 2.
Why is the dosing based on weight banding groups rather than an exact dose/kg?
Answer 2.
This is to reduce the increased error risk which can occur with volume administered based medications. This is also why the ampule volume has been rounded up to the nearest whole number.
Question 3.
Why is the SNAP protocol restricted to >6yrs patients and not for all patients?
Answer 3.
At the time of writing this guidance there is no data safety information for any patient under 6 years of age using the SNAP protocol. The RHC protocol may change to include this cohort if data becomes available reflecting the safe use of the SNAP protocol in under 6year olds.

