HHFNC systems appears to have similar effectiveness to nCPAP in improving clinical parameters in infants, but at flow rates above 2 L/min. HHFNC are thought to be more comfortable for preterm infants and to cause less nasal septal trauma than NCPAP. From the studies that have reported adverse outcomes, none found a significant increase or difference between HHFNC use vs CPAP use in risk of NEC, pneumothorax, BPD, IVH, ROP, days in hospital or on O2, sepsis or death 2. The use of HHFNC as a primary therapy from birth requires further research.
It is recommend only heated, humidified HFNC systems with flow rates >2 litres/min, up to the maximum flow rate recommended by the manufacturer are used 2. Prongs should not completely occlude the nares. Manufacturers recommend that the prong outer diameter occupy <50% of the nares internal diameter 9. When setting the flow rate, the infant’s weight must be considered as pressure generation increases with decreasing infant size 2. A starting flow rate of 4–6 L/min in newly born preterm infants appears to be a reasonable balance between efficacy and safety based upon the available evidence and current clinical practices.
The majority of units report weaning HHFNC by a gradual reduction in flow rate. Yoder et al 9 recommended increasing the flow rate in 1 L/min increments if (1) FiO2 increased by 0.1 above the starting FiO2, (2) pCO2 increased by 10 mm Hg (equivalent to 1.3kPa) above the baseline value, (3) increased distress or recession noted, or (4) decreased lung expansion noted on CXR. They recommended decreasing the flow rate by 0.5- to 1.0 L/min increments if all of the following were sustained for at least a 4-hour period: (1) FIO2 <0.3 and oxygen saturation within ordered parameters, (2) pCO2 was maintained within ordered parameters, (3) no signs of significant distress were noted, and (4) lung expansion was deemed adequate, if CXR was obtained.
Postulated mechanisms of action
Proposed mechanisms of action include elimination of dead space, reduction in work of breathing, improvement in lung compliance and delivering some degree of CPAP 2, 3.
- Washout of nasopharyngeal cavity
This is thought to be the primary mechanism, reducing the overall dead space and contributing to more effective CO2 elimination. Studies of comparable tracheal gas insufflation demonstrated reduced tidal volume and immediate effect on respiratory rate.
HHFNC reduction of the dead space also has an effect on oxygenation – due to reduced entrainment of room air, the airway oxygen concentration is higher when compared to use of non-rebreathing masks and in patients with mouth opened.
- Reduction of inspiratory resistance
HHFNC matches/exceeds the patient’s inspiratory flow and eliminates increasing nasopharyngeal resistance caused by its inspiratory distendability.
- Warm, humidified gas improves respiratory mechanics
Breathing cold, non-humidified gas for only five minutes decreases lung compliance and conductance. Cold, dry gas elicits bronchoconstriction (nasal mucosa receptors, muscarinic effect).
- Reduced metabolic cost of gas conditioning
The nasopharyngeal cavity provides effective warming and humidification to inspiratory gas but this requires a significant amount of energy. Babies on HHFNC have been shown to gain weight quicker than on CPAP.
- Provision of end distending pressure
High gas flow generates positive airway pressure (although very unreliably and depending on factors like body weight, mouth leaks, etc). It appears that larger babies require higher gas flow in order to maintain effective dead space washout and support inspiratory effort.
Potential concerns
The majority of units use HHFNC in babies of all weights and gestational age. Whilst this modality is popular, the limited evidence available does cause concern amongst clinicians about the exact pressures being delivered to these infant’s lungs and potential for overdistension and injury, particularly infants <1000g 3,4. Pharyngeal pressure delivered by HHFNC increases proportionally, in a linear fashion, when flow is increased 2,4. Pressure delivered to the baby is affected by infant’s weight (higher pressure with lower weights), size/calibre of the cannulae, proportion of nares occluded and if the mouth is open or not 1-4,6. According to more recent studies, pressures generated by HHFNC were similar to or less than those commonly set with nCPAP3. Flow rates of 8L/min have been shown to provide a distending pressure of 4-5 cmH20 7.The Vapotherm unit used in infants has a built-in pressure relief valve, will only deliver a maximal flow of 8L/min and will alarm if high pressure is detected in the circuit 4.
There was in the past a concern regarding potential for infections with Ralstonia spp and gram negative bacteria 1,2,3 which caused a recall of Vapotherm units in 2005. The Vapotherm infection control measures have since become more stringent. Vapotherm’s vapour transfer cartridges now have pores of 0.01 micrometers which are considerably smaller than bacteria (0.2-5micrometers) and thus form a barrier for these pathogens 5.
There has been a case report of a preterm infant who was receiving HHFNC at 2 L/min (device not reported) and concomitantly was found to have subcutaneous scalp emphysema and pneumo-orbitus, which resolved after discontinuation of the HHFNC 2,3. The authors noted that these complications may have been a result of nasopharyngeal trauma rather than the mode of support.




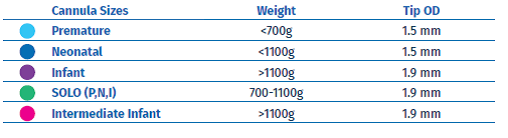

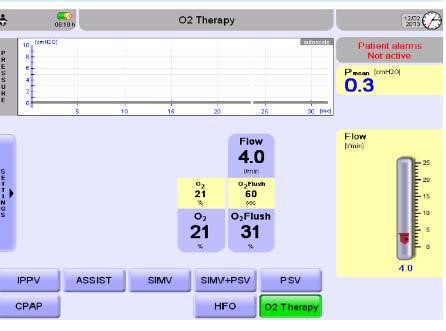
 Figure 2
Figure 2