Inhaled nitric oxide (iNO) is a useful adjunct in the treatment of neonates with hypoxaemic respiratory failure (HRF) due to persistent pulmonary hypertension (PPHN). PPHN may result from respiratory distress syndrome (RDS), meconium aspiration syndrome (MAS), sepsis and congenital diaphragmatic hernia (CDH) but in up to 10% of cases it is idiopathic.(1) A Cochrane review in 2017 concluded that iNO improves oxygenation and decreases the combined outcome of death or need for extracorporeal membrane oxygenation (ECMO) in term or near term infants. This reduction was due to a reduction in use of ECMO (with number needed to treat for an additional beneficial outcome (NNTB) of 5.3); mortality was not affected. (2) The review as with previous studies also concludes that the use of nitric oxide is not associated with any short term adverse effects or increased neurodevelopmental, behavioural or medical abnormalities at 2 years of age. (2,7)
Nitric oxide therapy in the neonate: guideline for the use of inhaled nitric oxide

Objectives
This guideline is applicable to all medical, nursing and midwifery staff involved in administering nitric oxide within the neonatal population in the West of Scotland. The guideline is to allow safe and consistent clinical practice of this therapy and is based on current best practice and evidence. The guideline is accompanied by an easy to follow set-up guide for the INOvent and NOxBOX systems.
Inhaled nitric oxide should be considered for any infant > 34 weeks gestation with hypoxaemic respiratory failure not responding to optimal mechanical ventilation who is suspected to have PPHN. Pre and post ductal saturations monitoring should be performed, a gap of > 10% signifies significant right to left shunting. Measures to optimise lung recruitment should be tried such as HFOV and surfactant administration. Correction of acidosis, anaemia and support of systemic blood pressure should also be carried out. An echocardiogram should be performed, if available, to look for signs of PPHN and rule out congenital heart disease as these infants can present with similar signs. Assessment of cardiac function can also be made guiding the most appropriate pharmacological management. Consideration should be given to starting Dinoprostone (Prostin) to maintain ductal patency and help offload the right heart in patients with severe PPHN.
Several randomised controlled trials and meta-analyses have failed to demonstrate the same benefit of iNO in preterm infants when compared to term and late preterm infants and should not be used routinely in this group of patients. (8-10) However, there is increasing evidence that in infants with a background of preterm premature rupture of membranes (PPROM), oligohydramnios and hypoxaemic respiratory failure that iNO may improve survival.(12-14)In a study by Aikio et al (13)it was shown that in preterm infants with respiratory failure following prolonged premature rupture of membranes (PPROM) there was a decrease in pro-inflammatory cytokines and low levels of nitrite and nitrate which increased in response to inhaled nitric oxide. This suggests that these infants may have a transient deficiency in the inflammatory response including a defect in nitric oxide generation in the airspaces. In this study the use of iNO acutely increased gas exchange, allowing for a decrease in both oxygen and ventilation requirements. A case series performed in one of the local tertiary NNU looking at preterm infants with a background of PPROM demonstrated a marked improvement in Oxygen Saturation following commencement of iNO. (15)
Preterm infants with a history of PPROM from < 24 weeks gestation or PPROM with severe oligohydramnios at 24 – 30 weeks gestation, who present with hypoxaemic respiratory failure, should be considered for iNO therapy. Delay in the availability of inhaled NO may reduce the infant’s chances of survival, or increase their morbidity. Therefore consideration should be given to antenatal transfer of any mother presenting with a history of PPROM from < 24 weeks, or PPROM with oligohydramnios from 24 – 30 weeks to a centre providing iNO therapy. WoS obstetric units have agreed a pathway to rebook mothers with a history of PPROM <24 weeks gestation at a tertiary unit offering iNO therapy.(see WoS Pathway for PPROM and oligohydramnios). The iNO should be commenced at 20ppm
The neonatal consultant should be informed before starting any infant on iNO. If in a unit that does not routinely use iNO any infant with on-going respiratory failure secondary to PPHN despite escalating treatment should be discussed early with the neonatal transport team to facilitate transfer to a centre that can provide iNO. In the meantime, ensure that factors below are optimised.
Before starting iNO optimise respiratory, cardiovascular and haematological/biochemical systems.
- Respiratory – Optimise lung recruitment, including HFOV
- Cardiac – Aim for systemic blood pressure at upper end of normal for gestation, generally accepted as mean blood pressure of +10mmHg above gestational age in weeks; consider Dinoprostone (Prostin)
- Haematology – Maintain Hb > 120g/L
- Biochemical – correct glucose, calcium and magnesium
- Acidosis – correct acidosis where possible
- Pharmacological –ensure adequate sedation and analgesia. Consider muscle relaxation in those who have failed to respond to other measures.
- Monitoring – pre and post ductal saturations, cardiac monitoring, invasive blood pressure monitoring
Ideally all infants should have an arterial blood gas and ECHO looking at TR jet prior to commencement of iNO. This allows monitoring of these markers in response to treatment. The iNO should be commenced at 20parts per million (ppm) in both term and preterm infants.
MetHb should be monitored 12 hourly (once per shift) and recorded on the blood gas results sheet. NO binds to haemoglobin and forms MetHb which prevents oxygen from binding to haemoglobin, resulting in reduced oxygen carrying capacity of the blood. Levels persistently above 2% should prompt assessment of the inhaled Nitric Oxide monitoring system and possible weaning of NO. In unlikely event that a baby suffers symptomatic methaemoglobinaemia, treatment with methylene blue may need to be considered.
Every hour
The following observations should be recorded every hour on the intensive care chart in addition to the standard observations of the ventilated neonate:
- Oxygen delivery indicated on iNO delivery device. (Each 10ppm of NO will displace ~ 2% of oxygen being delivered, so expect your ventilator set oxygen to be different).
- NO prescribed level which the iNO delivery device is set to deliver
- NO actual delivered level
- NO2 level in circuit
- Check water trap – empty if more than half full
- Check sample line – if blocked with water, use a new one
Every shift
- Check alarm limits are set appropriately – NO low alarm set at 5ppm less than prescribed dose and high alarm set 5ppm above set dose. NO2 alarm set at 2ppm
- A low calibration of NO, NO2 and O2 must be done and recorded (every 24hours)
- Check remaining cylinder level, ensure spare cylinder available and full
- Ensure methaemoglobin level done with blood gas
- Change filter on iNO delivery device, if saturated, or between patients
Weaning should be done cautiously due to the potential for rebound pulmonary hypertension, resulting in a dramatic drop in SaO2. This is due to down regulation of endogenous nitric oxide production that occurs during the administration of exogenous nitric oxide, resulting in a rebound vasospasm when the exogenous nitric oxide is withdrawn.(16) Weaning of iNO should ne commenced after a sustained improvement in oxygenation, usually enabling the oxygen to be reduced to less than 60%. Some infants will show an almost instantaneous response whilst others may take longer. Start by weaning the dose by 5ppm increments every 1 to 2 hours to 5ppm provided there is no significant deterioration in oxygenation. If weaning is unsuccessful then leave the baby on the lowest effective NO dose and retry after 12 hours if stable. Once down to 5ppm lower the dose in 1ppm increments every 1 to2 hours if no deterioration in oxygenation. Once stopped leave the nitric circuit attached to the ventilator for the next 24 hours until it is clear that the infant no longer requires therapy. Remember to turn the cylinder OFF and record patient details and cylinder number in the record of usage forms.
Fetal Circulation and Persistent Pulmonary Hypertension
In fetal life the lungs are relatively inactive and the placenta carries out the process of gas exchange. During this period the pulmonary vascular resistance (PVR) is high and directs blood away from the lungs to the rest of the body through the foramen ovale (FO) and the ductus arteriosus (DA, see figure 1). During and following delivery the fetal circulation must adapt so that the lungs are capable of taking over gas exchange. This occurs when the infant takes the first breath and oxygenation of the pulmonary vascular bed causes a decrease in PVR with subsequent increase in pulmonary blood flow. The increase in pulmonary blood flow causes the left atrial pressure to rise more than the right, functionally closing the FO. SVR increases when the cord is clamped thus removing the low resistance circulation of the placenta. As SVR rises above PVR the flow across the DA reverses and over the succeeding few hours after delivery the DA closes.
Figure 1. Fetal Circulation
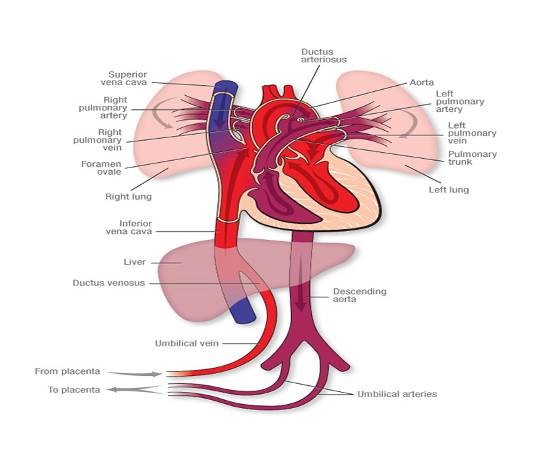
Figure 1. Fetal Circulation showing oxygenated blood returning from the placenta via the umbilical veins through the ductus venosus and bypassing lungs via the foramen ovale and ductus arteriosus. This oxygenated blood delivers oxygen to the rest of the body before returning to the placenta via the umbilical arteries.
PPHN occurs when PVR fails to fall and remains higher than SVR leading to right to left shunting of blood across the foramen ovale and ductus arteriosus resulting in hypoxaemia. (figure 2). PPHN can be a result of acute vasoconstriction in conditions such as MAS, asphyxia, sepsis and RDS; remodelling of the pulmonary vasculature in CDH, antenatal duct closure and chronic intrauterine hypoxia; intravascular obstruction in polycythaemia; or lung hypoplasia in CDH, intrathoracic space occupying lesion and chronic oligohydramnios. (1). It is believed that severe PPHN affects up to 2 to 6 per 1000 live births and complicates the course of 10% of all infants admitted to neonatal intensive care. It is responsible for an 8% to 10% risk of death and a 25% risk of long-term neurodevelopmental morbidity. (17)
Figure 2. Persistant Pulmonary Hypertension
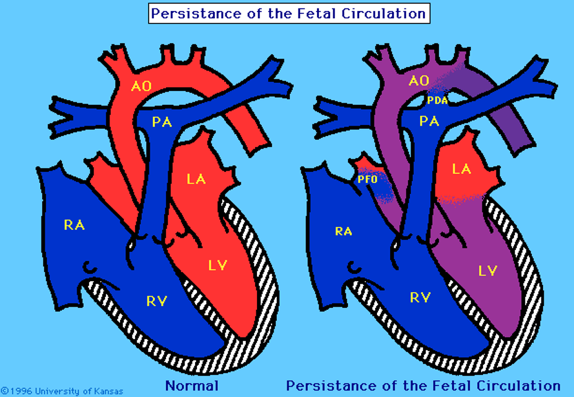
Figure 2. Demonstrates persistence of the foramen ovale and ductus arteriosus used in intrauterine circulation to direct blood away from the lungs.
Inhaled Nitric Oxide
The aim of PPHN therapy is selective pulmonary vasodilation. Before any pharmacologic treatment is commenced ventilation should be optimised to ensure adequate lung inflation and oxygen delivery. Achieving adequate lung inflation may require the use of high frequency oscillatory ventilation (HFOV) and exogenous surfactant administration, particularly in infants with parenchymal lung disease (eg. MAS, RDS). Correction of any acidosis is important as this can cause pulmonary vasoconstriction. Anaemia should be corrected to maximise oxygen carrying capacity but polycythaemia should be avoided as this can increase PVR. Cardiac function should be supported to optimise blood pressure, aiming for the high end of normal range as a minimum.
Nitric oxide (NO) is produced in the endothelium and readily diffuses into the vascular smooth muscle where through a series of pathways it stimulates pulmonary vasodilation. In PPHN endogenous NO production is reduced or dysfunctional.(17) Inhaled NO (iNO) is delivered to the respiratory tract via an endotracheal tube to result in pulmonary vasodilation. iNO interacts with haemoglobin to form methaemoglobin. (MHb). This reduces the oxygen carrying capacity of the blood and decreases the release of oxygen to the tissues so it is important that this is monitored while the infant it on iNO.

