Cenobamate guideline
exp date isn't null, but text field is
Objectives
Cenobamate is a novel antiseizure medication for the treatment of focal onset seizures in adult patients. Studies done in paediatric cohorts demonstrate similar efficacy rates and safety profiles on par with adult studies. There is evidence that Cenobamate can be used as an effective, safe, and tolerable adjunctive medication despite being currently unlicensed in children. This guidance is intended to support good practice around the prescription of Cenobamate and facilitate the audit of therapeutic trials.
Scope
This guideline is intended to apply for children between 10-18 years with drug-resistant epilepsy (failure of appropriately chosen 2 anti-seizure medications), who have been selected by a panel of paediatric neurologists (minimum of two). The guideline is not intended for children under 25kg of body weight. Children less than 10y of age and >25kg should only be prescribed Cenobamate if an Unlicensed Medication (ULM) request has been made and approved.
Audience
This guidance is targeted at paediatric neurology healthcare professionals. When implementing their judgement, healthcare professionals should consider the individual needs, preferences, and values of the patients and their legal guardians.
Criteria for initiation:
- Add on for pharmaco-resistant focal seizures in children between 10-18 years old who have failed in at least two anti-seizure medicines at appropriate doses.
- After discussion in an epilepsy consultants MDT meeting with at least two epilepsy consultants present.
- High seizure burden (multiple hospital admissions/ED attendances, unable to access education, injuries.)
- 4 weeks baseline seizure record, ideally through a prospective diary, prior to starting and monitoring of target seizure type whilst on Cenobamate treatment.
Treatment with Cenobamate is to be discontinued if:
- intolerable side effects.
- failure to improve target seizure type by at least 30% at 6 months.
Even though the mechanism of action is partly described it is postulated that it has a dual complementary mechanism of action. Cenobamate is a novel tetrazole-derived compound with one chiral centre. It reduces neuronal excitability by enhancing the fast and slow inactivation of sodium channels and preferentially inhibiting the persistent component of the sodium channel current. Moreover, cenobamate is a positive modulator of high-affinity GABA, acting through allosteric changes from binding at a non-benzodiazepine site.
Cenobamate 12.5 mg tablets and 25 mg film-coated tablets. Initiation pack (14+14)
Cenobamate 50 mg film-coated tablets (28)
Cenobamate 100 mg film-coated tablets (28)
Cenobamate 150 mg film-coated tablets (28)
Cenobamate 200 mg film-coated tablets (28)
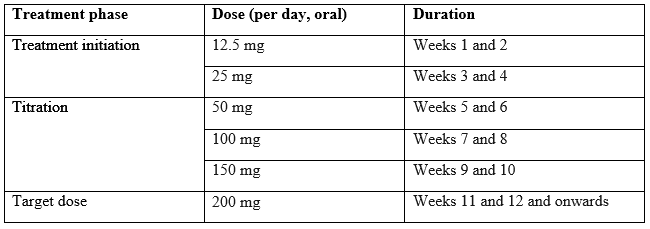
The recommended starting dose of Cenobamate is 12.5mg given once per day, typically in the evening. The dose should be titrated slowly to the recommended target dose of 200 mg per day.
Some patients, who do not reach optimal seizure control, may benefit from optimising the doses above 200 mg (increased by increments of 50 mg/day every two weeks) up to a maximum of 400 mg daily however this dose. The maximum upper limit for individuals <50kg is 4.5mg/kg/d.4
The recommended titration schedule is provided in table below.

Missed doses
If patients miss one dose, it is recommended that they take a single dose as soon as they remember, unless it is less than 12 hours until their next regularly scheduled dose.
Discontinuation
It is recommended that discontinuation be undertaken gradually to minimize the potential for rebound seizures (i.e., over at least 2 weeks) unless safety concerns require abrupt withdrawal.
Renal impairment
Should not be used in children with end-stage renal disease or patients undergoing haemodialysis.
Should be used with caution and reduction in the target dose in children with mild, moderate, or severe renal impairment. The maximum Cenobamate dose in the above scenarios is 300mg per day.
Hepatic impairment
Exposure to Cenobamate was increased in patients with chronic hepatic disease. No change in the starting dose is required but a decrease in target doses up to 50% may need to be considered. The maximum recommended dose in patients with mild to moderate hepatic impairment is 200 mg per day. It should not be used in patients with severe hepatic impairment.
- Currently available only for oral use.
- Should typically be taken once daily as a single dose at any time, preferably be taken at the same time each day.
- May be taken with or without food.
- Tablets are film coated and should be swallowed whole (12.5mg tablets are not film coated)
- Do not cut tablets as there is no break line and the accuracy of the dose cannot be guaranteed.
- The film-coated tablet may be crushed and dispersed in water in tube-fed children.
- Patients with galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take the tablet.
Caution
- Suicidal ideation: patients should be monitored for signs of suicidal ideation and behaviours and appropriate treatment should be considered
- Drug reaction with eosinophilia and systemic symptoms (DRESS): If signs and symptoms suggestive are present Cenobamate should be withdrawn.
- Mild to moderate hepatic or renal impairment
- Co-administration with other medications known to cause short QT interval.
- Women of childbearing age must use effective contraception while on Cenobamate treatment and 4 weeks after its discontinuation. The efficacy of hormonal contraception may be reduced by Cenobamate, hence non-hormonal contraceptive methods are advised.
- Children with a body weight of under 25 kg
Contra-indication
- Familial short QT syndrome
- End-stage renal and hepatic failure
|
Frequency |
Adverse Reactions from clinical trials |
|
Common or very common |
Confusion; constipation; diarrhoea; dizziness; drowsiness; dry mouth; fatigue; gait abnormal; headache; hepatic function abnormal; hypersomnia; irritability; memory impairment; movement disorders; nausea; nystagmus; skin reactions; speech impairment; vertigo; vision disorders; vomiting |
|
Rare |
Drug reaction with eosinophilia and systemic symptoms (DRESS) |
|
Frequency unknown |
Suicidal behaviours |
Extensively metabolized by glucuronidation via UGT2B7 and to a lesser extent by UGT2B4. Also metabolized by CYP isoenzymes
Drugs metabolized by hepatic microsomal enzymes
CYP2B6 and CYP3A substrates: Cenobamate may decrease plasma concentration and efficacy of substrate drug. Consider an increase of substrate drug as needed.
CYP2C19 substrates: Cenobamate may increase the plasma concentrations of the CYP2C19 substrate, potentially increasing the risk of adverse reactions. Consider decreasing the dosage of substrate drug clinically appropriate if adverse reactions are observed.
Drugs that shorten QT interval
Potential additive effect on QT interval. Use concomitantly with caution.
Specific drug
|
Drug |
Interaction |
Comments |
|---|---|---|
|
Alcohol |
This may increase the risk of sedation and somnolence |
|
|
Carbamazepine |
Peak plasma concentration and AUC of carbamazepine each decreased by 23% |
Increase Carbamazepine dose as clinically appropriate |
|
Clobazam |
Increase plasma concentration of active metabolite of clobazam. (desmethylclobazam) |
Consider a reduction in the Clobazam dosage |
|
CNS depressants |
This may increase the risk of adverse neurological effects including somnolence and sedation. |
|
|
Lacosamide |
Clinically important effects are not observed. |
|
|
Lamotrigine |
Lamotrigine concentrations are expected to decrease by 21-52%. Possible adverse effects on shortening QT interval. |
Increase the Lamotrigine dose as clinically appropriate. Be cautious in co-administration |
|
Levetiracetam |
Clinically important effects are not observed. |
|
|
Midazolam |
Peak plasma concentration and |AUC decreased by 61% and 72% respectively. |
Increase midazolam dosage as needed. |
|
Omeprazole |
Peak plasma concentration and |AUC increased by 83% and 107% respectively. |
Decrease omeprazole dosage as clinically appropriate if adverse effects occur |
|
Oral Contraceptives |
May decrease the concentration and efficacy of OCP |
Use additional or alternative non-hormonal product |
|
Oxcarbazepine |
Interaction unlikely |
|
|
Phenobarbital |
Increased phenobarbital plasma concentration and AUC 34% and 37% respectively. |
Consider dosage reduction of phenobarbital as clinically appropriate |
|
Phenytoin |
Increased phenytoin peak plasma concentration and AUC by 70% and 84% respectively and Cenobamate exposure decreased by 27-28% |
Gradually reduce phenytoin dosage by up to 50% as Cenobamate is being titrated |
|
Primidone |
Possible additive effect on shortening of QT interval |
Use concomitantly with caution |
|
Rufinamide |
Possible additive effect on shortening of QT interval |
Use concomitantly with caution |
|
Valproic acid |
No effects of the pharmacokinetics of Valproic acid |
|
|
Warfarin |
No clinically important effects on Pharmacokinetics of Warfarin |
|
- 12 lead ECG at baseline and repeat if necessary.
- Baseline blood tests (FBC, LFTs, and blood urea and electrolytes) and repeat these after 1 and 3 months of treatment and thereafter only if clinically indicated.
- Monitor for signs of suicidal ideation and behaviour.
Cenobamate is unlicensed for children. The usage of Cenobamate in the Royal Hospital for Children in Glasgow will be funded by NHS Greater Glasgow and Clyde.
Epilepsy Nurse Specialist Team – 0141 4524036
Angelini Pharma UK-I Limited. Cenobamate 50 mg film-coated tablets. Summary of Product Characteristics UK. [Internet]. Last updated: Aug 2022 [cited Jan 2023]. Available from: https://www.medicines.org.uk.
British National Formulary: Cenobamate Monograph. [Internet] Via Medicines Complete. Last updated March 2022 [cited Jan 2023]. Available from: https://www.medicinescomplete.com.
Cenobamate for treating focal onset seizures in epilepsy-Technology appraisal guidance [TA753] Published: 15 December 2021. [cited Jan 2023]. Available from: https://www.nice.org.uk/guidance/ta753
Varughese RT, et al. Adjunctive use of cenobamate for paediatric refractory focal-onset epilepsy: A single-centre retrospective study. Epilepsy Behav. 2022;130:108679
Elliott T et al. Initial Real-World Experience With Cenobamate in Adolescents and Adults: A Single Center Experience. Pediatr Neurol. 2022;129:19-23
Ferrari L, et al. An Ex Vivo Evaluation of Cenobamate Administered via Enteral Tubes. Drugs R D. 2020;20(2):125-133
Steinhoff BJ, Rosenfeld WE, Serratosa JM, Brandt C, Klein P, Toledo M, Krauss GL. Practical guidance for the management of adults receiving adjunctive cenobamate for the treatment of focal epilepsy-expert opinion. Epilepsy Behav. 2021 Oct;123:108270. doi: 10.1016/j.yebeh.2021.108270. Epub 2021 Sep 8. PMID: 34509033.
Sperling, MR, Abou-Khalil, B, Aboumatar, S, Bhatia, P, Biton, V, Klein, P, et al. Efficacy of cenobamate for uncontrolled focal seizures: Post hoc analysis of a Phase 3, multicenter, open-label study. Epilepsia. 2021; 62: 3005– 3015. https://doi.org/10.1111/epi.17091
NHS Greater Glasgow and Clyde: New Medicines Decisions February 2022.
Last reviewed: 02 April 2024
Next review: 28 April 2027
Author(s): Professor Sameer Zuberi
Version: 1
Author Email(s): sameer.zuberi@ggc.scot.nhs.uk
Co-Author(s): Dr Thilina Munasinghe
Approved By: Department of Paediatric Neurololgy
Reviewer Name(s): Professor Sameer Zuberi

