Management and investigation of bloody diarrhoea and haemolytic uraemic syndrome
exp date isn't null, but text field is
Objectives
The following guideline has been developed and is regularly reviewed by clinicians within the Renal Unit at the Royal Hospital For Children (RHC), Glasgow. These guidelines are based on current evidence and best practice relating to the Investigation and Management of Haemolytic Uraemic Syndrome and reflect the Health Protection Scotland Guideline Recommendations. This document is intended for use by clinicians and nursing staff in the treatment of Haemolytic Uraemic Syndrome. For further discussion of this guideline, please contact a consultant within the Renal Unit. Please contact the author prior to any duplication of the protocol.
Haemolytic Uraemic Syndrome (HUS) is the commonest cause of intrinsic renal acute kidney injury in children in Scotland. In most cases, it follows infection with shigatoxin producing Escheriae Coli (E.Coli), the commonest subtype being 0157:H7. In most patients, there is a preceding history of diarrhoea which is frequently bloody. 10-15% of patients will progress to develop HUS, with children <16 years (in particular patients <5 years) at highest risk.
Haemolytic Uraemic Syndrome refers to a triad of findings which include:
- Microangiopathic Haemolytic Anaemia
- Red cell fragmentation on Blood Film
- Acute Kidney injury
Acute bloody diarrhoea should be treated as a medical emergency and requires urgent action. Patients in primary care should be referred for urgent same day paediatric assessment and triaged at minimum category 3. All patients will require paediatric assessment and bloods and urinalysis at first assessment.
The incubation period of E.Coli is typically 3-4 days however may be as long as 14 days. The mean onset of HUS to develop after initial diarrhoea is 7 days. Normal blood results during the diarrhoeal phase do not exclude HUS and it is important to take a clear history of onset of diarrhoea for risk assessment.
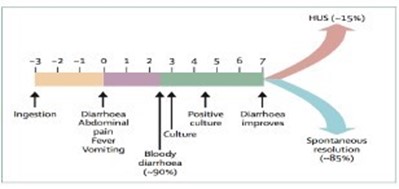
Figure 1: Timeline of STEC-HUS: Days from ingestion to symptoms12
HUS most commonly follows a diarrhoeal prodrome but may also occur after infections with other organisms such as S. Pneumoniae, or from other aetiologies such as drugs, pregnancy or may be familial. These other forms of HUS are mentioned in Section 7
Criteria for entry
Criteria for entry into the pathway for Bloody Diarrhoea or suspected E.Coli are:
- Acute bloody diarrhoea
- Minimum of one episode of blood in stool AND
- Diarrhoea defined as acute onset loose stool
- Non bloody acute diarrhoea AND suspicion of Coli
- Contact with
- farm animals
- contaminated environments (fields, farms, rural areas)
- Untreated water from rivers or private supplies
- A known or suspected case of E.Coli
- Contaminated food (undercooked meat, unpasteurised milk, raw vegetables)
- Travel out with the UK
- An outbreak of Coli is known to be present locally or nationally
- Contact with
Other causes of blood in stools should always be considered, including surgical causes.
Patients with blood in stools who should NOT directly enter the pathway unless specific concerns of exposure to E.Coli include:
- Infants and children with suspected cow’s milk protein intolerance
- Patients with suspected or confirmed inflammatory bowel disease
- Patients with blood coated stool secondary to constipation, including those with overflow diarrhoea
It is recommended all patients with blood in the stool have stool cultures sent to exclude E.coli
Patients should be assessed urgently and a standard assessment of Airway, Breathing and Circulation should be performed. A full history and examination should be undertaken, with attention paid to the patients’ intravascular status by assessing: -
|
|
Dehydration should be identified early and treated promptly with intravenous 0.9% Sodium Chloride rather than oral rehydration. Hypovolaemia should be treated by volume expansion with 0.9% Sodium Chloride, 4.5% Human Albumin Solution or Packed Red Blood cells depending on clinical status, with full fluid management detailed below.
Any patient with bloody diarrhoea should be considered as infectious and infection control measures put in place, including strict hand hygiene in primary caregivers. Person to person spread is common, therefore other family members, in particular children under the age of 16 years and adults >65 years or with other co-morbidities should also be identified for assessment locally. This should be facilitated in conjunction with Public Health and done before stool result is known if the diarrhoea is bloody or E.Coli is suspected.
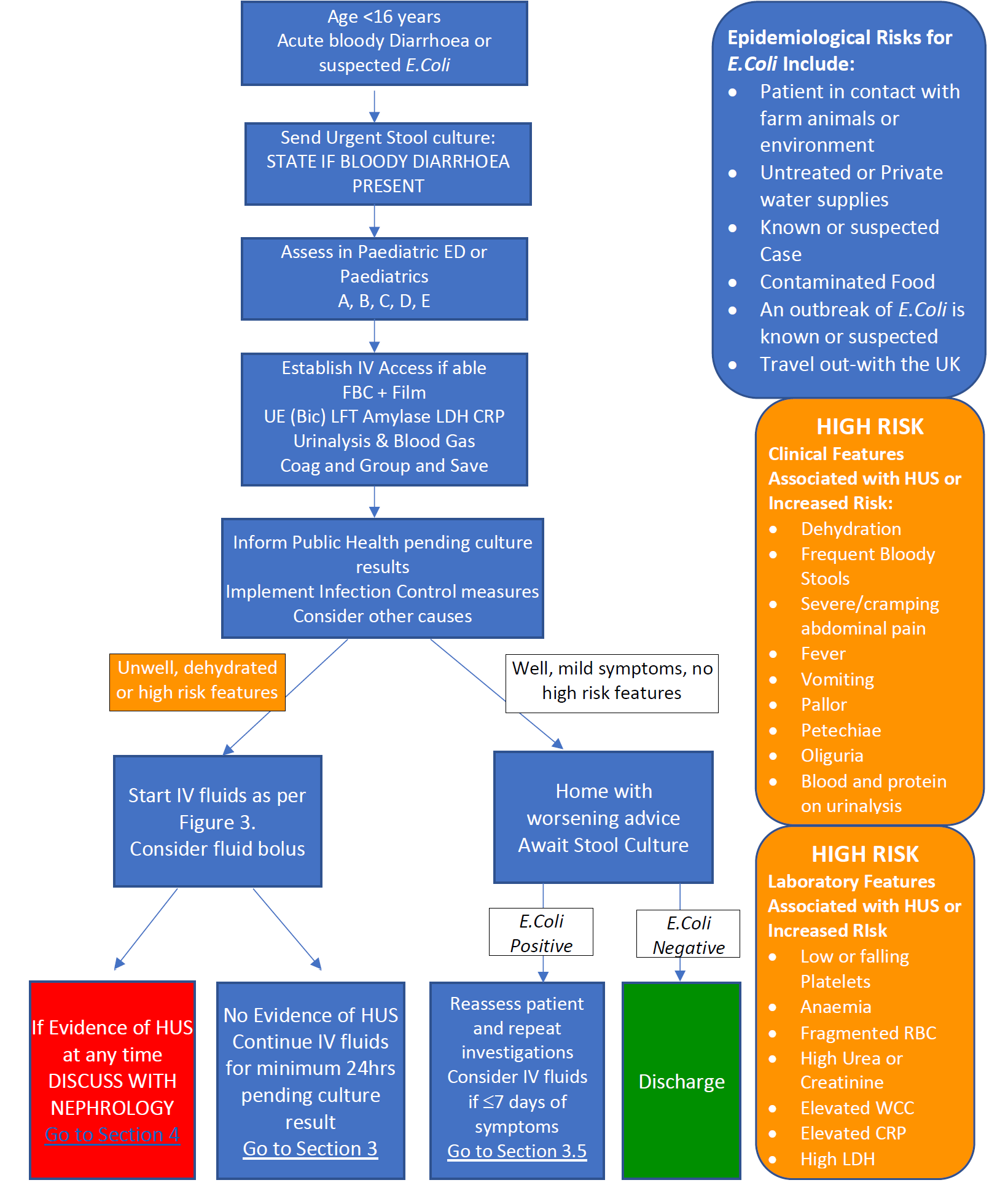
2.1 Initial Investigations of Bloody Diarrhoea or Suspected E.Coli
- Establish IV Access if possible whilst taking the following bloods on ALL patients:
- Full Blood Count with film and differential
- U&Es, LFT’s, Amylase, LDH, CRP, Bicarbonate and Glucose
- Stool culture – URGENT sample
- Please state if bloody diarrhea is present on the request form
- Group and Save
- Note local healthcare policy: GG&C require two separate samples to be taken from May 2014 before blood products issued
- Blood products can be matched from one sample in emergencies; this MUST be discussed with the laboratory
- Coagulation Screen
- E.Coli serology (clotted sample to microbiology)
- Urinalysis
- Urine Culture
- Pregnancy test in females >12 years of age or of child-bearing potential if HUS confirmed
- Other investigations as clinically indicated e.g. CXR, Renal Ultrasound
If the patient does not have investigations or clinical signs suggestive of HUS please go to Section 3: Management of Bloody or E.Coli positive diarrhoea without HUS.
If the presentation and investigations suggest HUS, please continue to Section 4: Management of Suspected HUS below.
Assessment and initial management should be carried out as described in Section 2. Initial Assessment and Management
There is preliminary evidence that IV 0.9% Sodium Chloride prevents development of HUS and reduces progression to oligo/anuria when commenced within 4 days of diarrhea onset or at the earlier stages of HUS. Early initiation is important. Oral rehydration has not been shown to be nephroprotective, therefore good oral intake should not replace the role of IV fluids.
It is recommended any child with bloody diarrhea or suspected or confirmed E.Coli positive stools that are:
- within 7 days of initiation of symptoms AND
- with high risk features (clinical or biochemical Table 1) AND
- with NO signs of HUS
- Are commenced pre-emptively on IV 0.9% Sodium Chloride for a minimum of 24 hours.
- There should NOT be a delay in fluid administration whilst waiting for an Coli stool result.
Note: If patients continue to be symptomatic and have high risk features but are >7 days of symptoms, consider for IV fluid therapy as mean onset of HUS is 7 days but can occur up to day 14.
Care of patients with Bloody Diarrhoea or E.Coli positive stools receiving protective IV fluids should initially be led by the local consultant and do not initially require discussion with the on-call Nephrology consultant unless there are signs of HUS or fluid overload.
3.1 Fluid Management
Fluid Management is dependent on the patient’s current hydration status. In the presence of clinical fluid overload fluids should be administered cautiously and this should be discussed initially with the Local Consultant on call. However it is more common for hypovolaemia or euvolaemia to be present. Hypovolaemia at presentation with E.Coli is associated with increased risk of severity of HUS if disease progresses. It is important to treat promptly.
|
Patient history |
Exam |
Laboratory findings |
|
Thirst |
Cool peripheries |
Increased haemoglobin |
|
Oliguria |
Reduced CRT |
Increased haematocrit |
|
|
Skin Turgor |
Hypercalcaemia |
Figure 2. Signs of hypovolaemia
Patient weight can be critical in this assessment and wherever possible a patient should be weighed at presentation and compared to previous measurements.
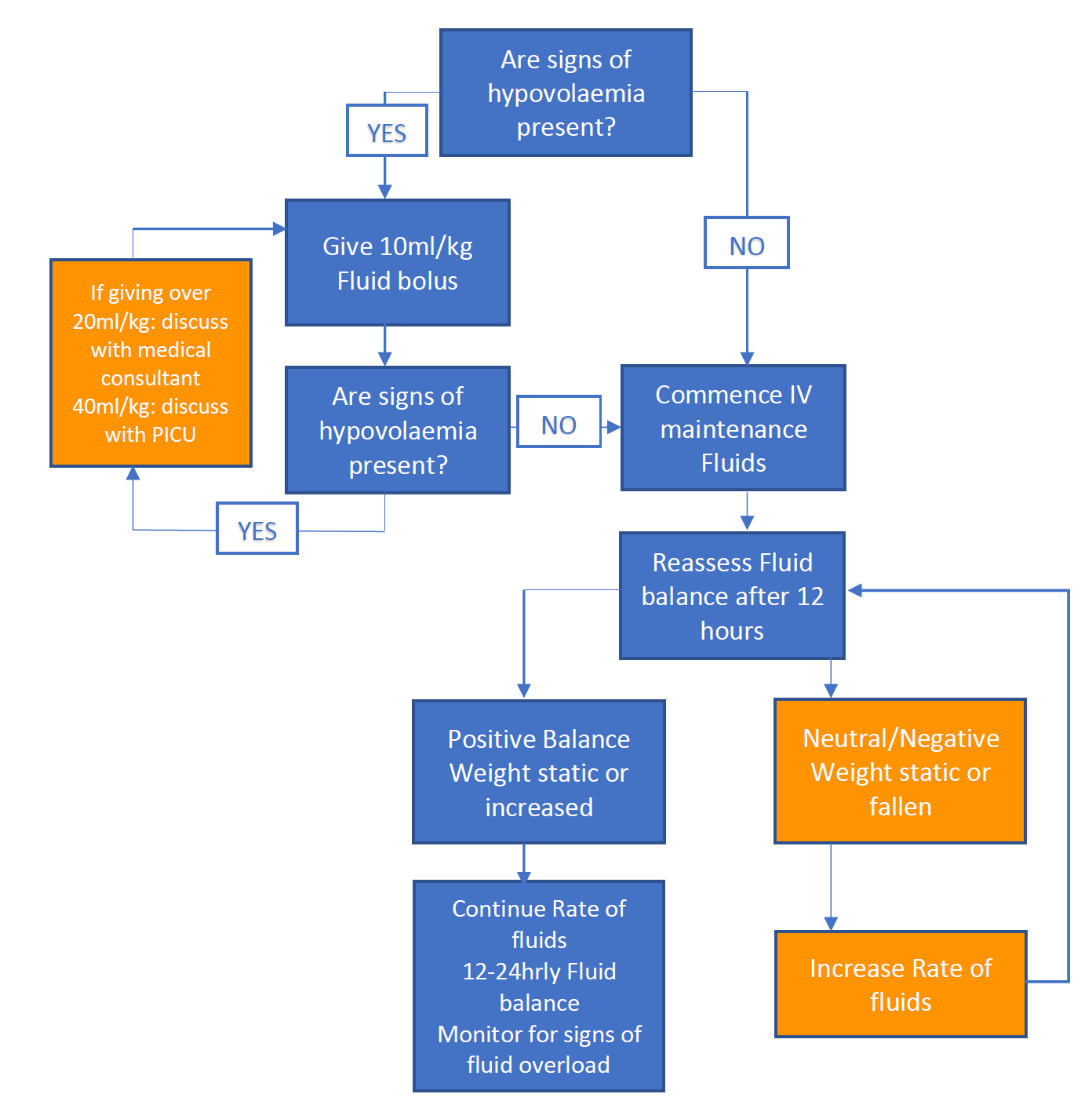
In the presence of hypovolaemia, fluid boluses of;
- 0.9% Sodium Chloride or 4.5% Albumin can be safely given in aliquots of 10ml/kg (max 500ml per bolus)
- Patients response should be measured and documented after each bolus by assessing signs of hydration, paying attention to heart rate and oxygen saturations.
- This can be repeated to a maximum of 20ml/kg. Further fluid boluses should be discussed with the Local Consultant and consider discussion with the Consultant Paediatric Nephrologist.
- Any child requiring 40ml/kg of fluid or more should be discussed with or reviewed by PICU.
- Assuming adequate resuscitation fluid, patients should then be managed as for euvolaemia below.
- Some children may be oedematous but still intravascularly deplete
Euvolaemia
If euvolaemia is present at first assessment or following fluid bolus,
- IV fluids should be started at maintenance rate with 0.9% Sodium Chloride. 0/9% NaCl and 5% Dextrose can be used if the patient is not eating.
- Potassium can be added to the bags as appropriate as hypokalaemia is common, however should continue to be monitored on UE with renal function and urine output.
- Patients may be allowed to drink in addition if fluid balance and weight are carefully monitored.
- Patients should maintain a positive fluid balance in the context of euvolaemia and ongoing stool losses may require an increase in IV fluids above maintenance rate.
- IV fluids are the priority and oral fluids should not be used as a substitution of the recommended IV rate.
Fig.3 Fluid administration in patients at risk of HUS
3.2 Monitoring during IV Fluid therapy without signs of HUS
Patients should have a minimum of
- Weight – daily
- Fluid Balance – Assessed 12-24 hourly with accurate input/output
- 6 hourly Heart Rate and Blood Pressure
- Bloods – Daily: UE, LFT, LDH, Amylase, CRP and FBC with film
- Urinalysis – Daily: haematuria and proteinuria can indicate development of HUS
3.3 Stopping IV hydration
NOTE: The decision to stop IV fluids lies with the team looking after the child and should be based on hydration status and clinical status.
If the stool culture returns NEGATIVE for E.Coli and clinically E.Coli infection is not suspected, IV fluids can be stopped for prevention of HUS.
If the stool culture returns POSITIVE AND after a minimum of 24 hours of IV Fluids:
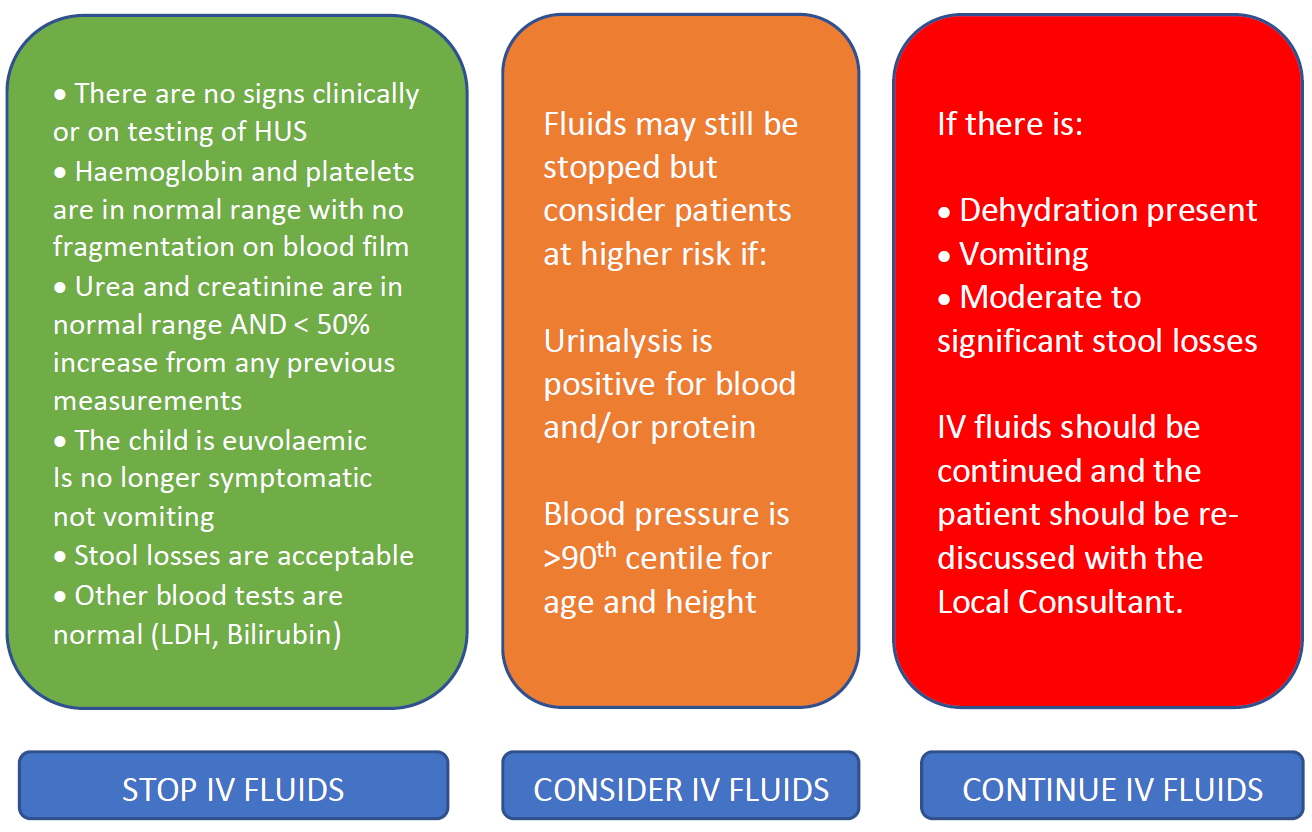
The patient can be discharged home and followed up as an outpatient until 14 days after commencement of diarrhea or symptoms as per the follow up flow diagram below.
If a child at any time develops signs suggestive of HUS they should be discussed with
Paediatric Nephrology and managed as per Section 4: Management of Suspected HUS
3.4 Monitoring after discharge
Patients should be followed up until day 14 for potential development of HUS by the local paediatric team, or where appropriate the general practice following discussion.
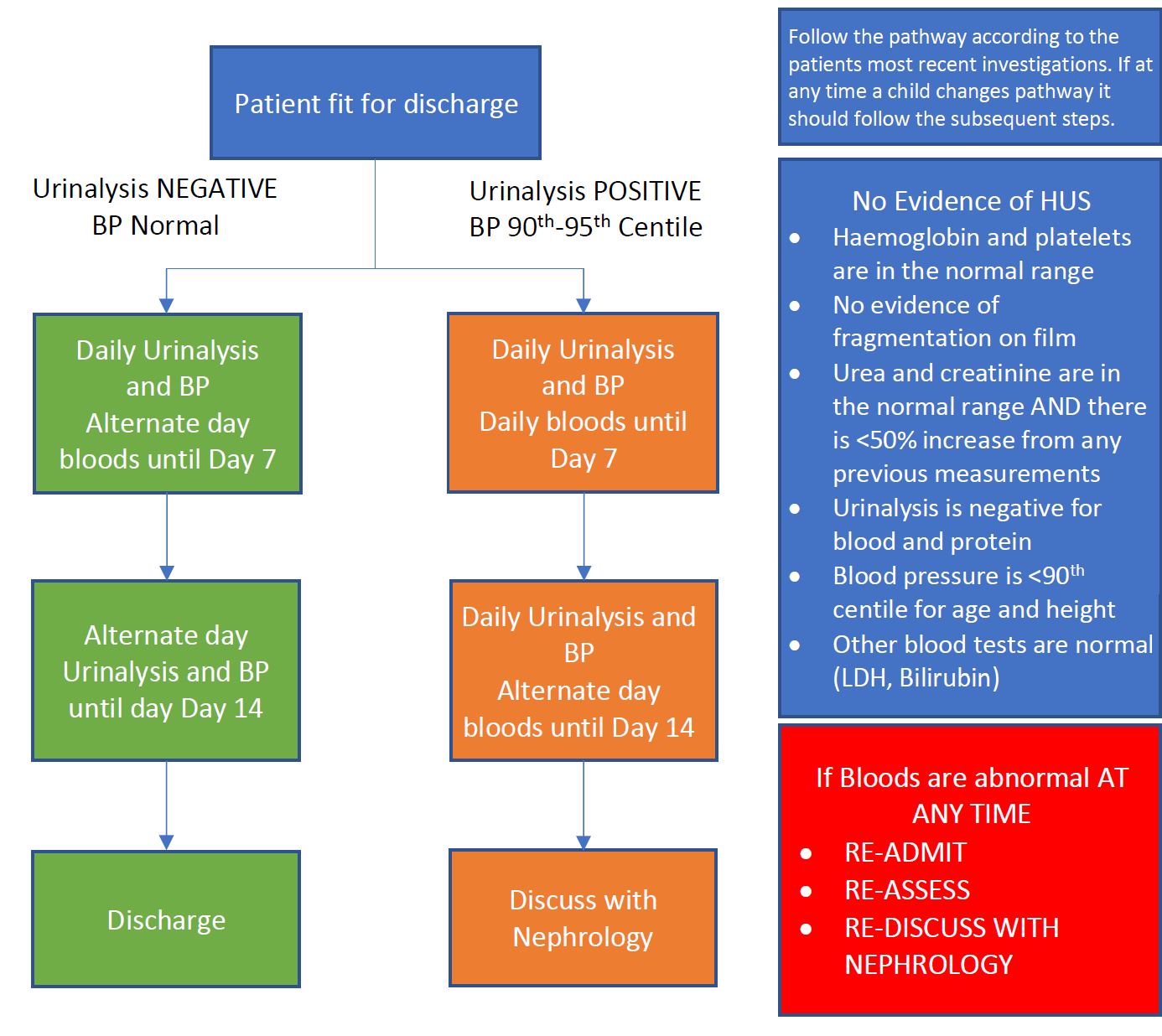
3.5 Children initially assessed or E.Coli confirmed after 7 days of commencement of symptoms
Children assessed after 7 days of initiation of diarrhea who are either E.Coli positive or suspected to have E.Coli should have all investigations performed as in Sections 2. Initial Assessment and Management
If after full assessment with no signs clinically or on testing of HUS all the criteria above in Section 3.3 are fulfilled, the patient can be discharged home and followed up as an outpatient until 14 days after commencement of diarrhea or symptoms as in Section 3.4 Monitoring after discharge
Note: If patients continue to be symptomatic and have high risk features but are >7 days of symptoms, consider for IV fluid therapy as mean onset of HUS is 7 days but can occur up to day 14.
If the presentation and investigations suggest HUS, please discuss with local consultant on call and Nephrology consultant on call in RHC for further management advice including fluid management.
A list of desirable information at time of referral is attached (Appendix A)
Parent and patient information sheet can be found at http://www.infokid.org.uk/STEC-HUS
4.1 Fluid Management in HUS
In the context of Oligo/Anuria or Fluid Overload following discussion:
Fluid should be administered cautiously and should not exceed the insensible fluid losses plus the urine output. Insensible losses should be based on body surface area.
Urine output can be measured and replaced hourly if a catheter is in situ, however this is not often necessary. Measuring 4 hourly output then replacing over the next four hours is often practical. Output includes all losses (diarrhoea, urine, drain losses)
Insensible Fluid Calculation= 400ml per m2 Body Surface Area in 24 hours
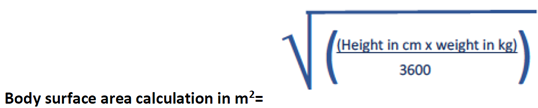
Where height is not available due to patient condition, please refer to the body surface area table based on weight in the latest edition of cBNF.
|
For example, a 12kg height 110cm child passes 80ml of urine in a 4-hour period. Fluids were running at 48ml/hr. The child has passed 40ml of diarrhoea Insensible fluids calculation = 400ml x0.6m2 in 24h= 240mls/24 hour s= 10mls/hr Urine output = 80ml in 4h = 20ml/hr Stool output = 40ml in 4h = 10ml/hr Total fluids per hour for next four hours are= 10 + 20 + 10 = 40ml/hr |
Further information in insensible fluid prescribing can be found in the guideline: Investigation and Management of Acute Kidney injury.
4.2 Monitoring in suspected HUS
Minimum recommended monitoring for patients with HUS include:
- Weight – minimum daily
- Fluid Balance – Assessed 6 hourly with accurate input/output
- 4-6 hourly Heart Rate and Blood Pressure
- Bloods: Daily – UE (Bic), LFT, LDH, Amylase, CRP, FBC with film
- Glucose daily – patients are at risk of pancreatic involvement and may develop hyperglycaemia
- Urinalysis – daily
- Weekly protein/creatinine ratio
- Active Group and Save
4.3 Electrolyte Abnormalities
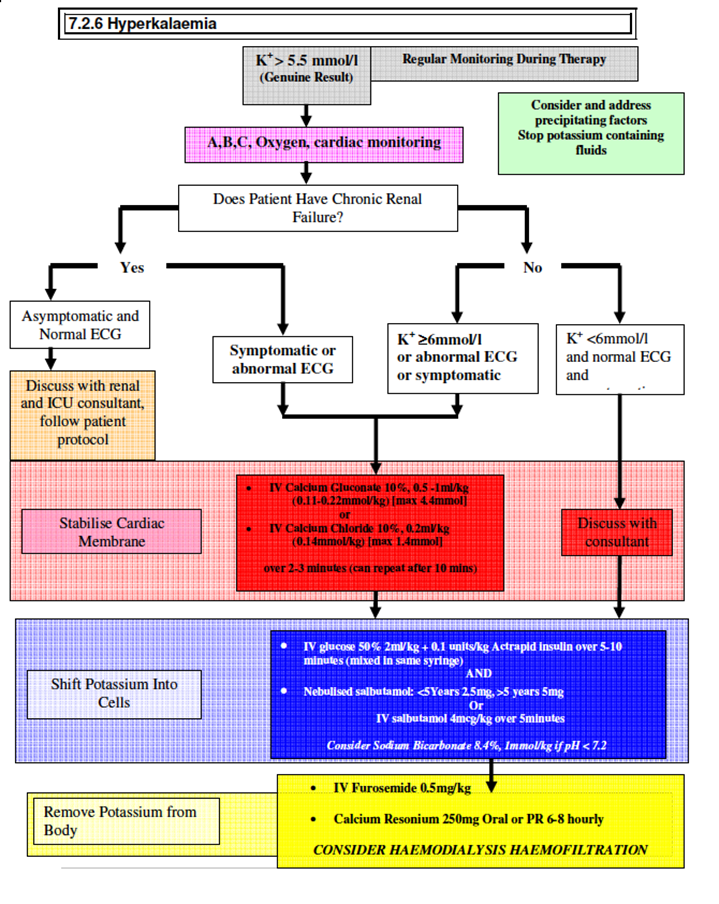
Full details of electrolyte abnormalities in patients with Acute Kidney injury are found in the protocol; Investigation and Management of Acute Kidney injury, however the hyperkalaemia guideline is attached in Appendix C.
Hyponatraemia needs to be assessed in association with fluid status and managed appropriately.
4.4 Altered Consciousness or Focal Neurology
Neurological involvement is not uncommon in HUS, and may be associated with a significant rise in morbidity and mortality. Symptoms may be mild such as irritability or mild encephalopathy and should be monitored and discussed with the tertiary centre.
Any change or deterioration should prompt a full and urgent neurological exam should be performed with immediate discussion with the responsible senior clinician. Neuro observations should be commenced. Consider urgent neuro-imaging, including out-of-hours.
If a patient is seizing, the APLS guidelines for seizure management should be undertaken and discussion with PICU for consideration of neuroprotective management.
4.5 Hypertension
Hypertension is often present in patients with HUS and requires careful patient assessment and may be secondary to fluid overload, pain or intrinsic due to HUS.
If the patient is not responsive to diuretics and fluid cannot be removed timely or safely with dialysis, they may benefit from a vasodilator such as amlodipine.
Hypertension should be discussed with the Nephrology Consultant on Call prior to initiation of medication as mild hypertension may be tolerated. Further information can be found in the guideline; Investigation and Management of Acute Kidney Injury
4.6 Abdominal pain/vomiting
Abdominal pain is commonly due to the colitis that occurs in diarrheal HUS and can be pronounced around defecation. Initial treatment should be with paracetamol as required every 6 hours if renal impairment present.
It may be necessary to use opiate analgesia however these should be started at a low dose and titrated to response. In the presence of renal impairment opiates will accumulate and response needs to be regularly reassessed. Pharmacy advice should be sought for prescription of opiates.
DO NOT PRESCRIBE IBUPROFEN
4.7 Nutrition
All patients should be referred to a paediatric dietitian. A nasogastric tube should be inserted at the time dialysis access is obtained or prior if caloric intake is poor. Feeds should be directed by the paediatric dietitian. Choice of feed is determined by patients’ biochemistry and dialysis and volume may be restricted either orally or via NG.
4.8 Role of Antibiotics in Children with Suspected or Confirmed E.Coli Infection
In children with suspected or confirmed E.Coli infection antibiotics are not recommended. There is evidence that antibiotics, in particular β-lactams may be a risk factor for HUS.
The use of antibiotics should, therefore, be governed by good practice as indicated by clinical needs other than the management of STEC infection itself.
- Fluid overload resistant to diuretics
- Hyperkalaemia
- Intractable Acidosis
- Symptoms of uraemia
- Likely progression to one of the above
5.1 Choice of Dialysis Modality
Most patients will be commenced on peritoneal dialysis except those with severe colitis, cerebral HUS or profound metabolic abnormalities.
This decision should be made following consultation with the duty nephrologist
5.2 Peritoneal Dialysis
The Paediatric Nephrology Consultant will contact the on-call Surgical Consultant and request placement of a peritoneal dialysis catheter. The surgical team will follow current best practice in fixation of the PD catheter due to the acute requirement of dialysis.
In addition, contact Anaesthetics to insert a small percutaneous double lumen external jugular line for sampling) and a nasogastric tube of appropriate size. Femoral lines are not appropriate for sampling. (See protocol entitled – Acute Peritoneal Dialysis)
|
Peritoneal Catheter Size |
|
|
Patient Weight |
Catheter and Size |
|
Neonatal < 5Kg |
31cm |
|
3.5 - 7.5Kg |
39cm curlcath |
|
7.5 - 20Kg |
57cm curlcath |
|
>20Kg |
60cm curlcath |
Prior to theatre
- Ensure patient cross-matched for 2 units of packed red cells
- Discuss platelet count and requirements with on-call surgical team
- Obtain up to date UE, LFT, and FBC prior to theatre
- Select the appropriate PD catheter and NG tube
Antibiotics should not be given for most patients having PD catheter insertion unless specifically instructed by the Nephrology Consultant on call. This is due to the adverse effects reported with antibiotic administration in HUS.
Dialysis prescription should be based on the protocol entitled – Acute Peritoneal Dialysis
5. 3 Haemodialysis
Haemodialysis is indicated for those with severe colitis, cerebral HUS or those with profound metabolic derangements such as hyperkalaemia, hyperuricaemia or markedly catabolic (See protocol entitled – Acute Haemodialysis)
The Paediatric Nephrology Consultant will contact the on-call Surgical Consultant and request placement of a haemodialysis line. In addition, contact the Anaesthetist and ask them to place a nasogastric tube under anaesthetic.
Antibiotics should not be given for most patients having HD catheter insertion unless specifically instructed by the Nephrology Consultant on call. This is due to the adverse effects reported with antibiotic administration in HUS.
|
Haemodialysis Access Internal Jugular Lines |
|||||||||||||
|
Patient Weight |
Type of Line |
Size |
Total |
Tipcuff |
|
Prime Volume (ml) |
|||||||
|
Art. |
Venous |
||||||||||||
|
5-10kg |
Medcomp |
8FG X 12cm |
12cm |
|
0.6 |
0.6 |
|||||||
|
5-10kg |
Kflow Epic |
10FG X 18cm |
18cm |
13cm |
0.5 |
0.6 |
|||||||
|
10- 15kgs
|
Kflow Epic |
12FG X 16cm |
21cm |
16cm |
1 |
1.1 |
|||||||
|
10- 15kgs |
Kflow Epic |
12FG X 19cm |
24cm |
19cm |
1.05 |
1.1 |
|||||||
|
15- 30kgs |
Kflow Epic |
14FG X 19cm |
24cm |
19cm |
1.55 |
1.65 |
|||||||
|
15- 30kgs |
Kflow Epic |
14FG X 23cm |
28cm |
23cm |
1.65 |
1.7 |
|||||||
|
30- 40kgs |
Kflow Epic |
14FG X 27cm |
32cm |
27cm |
2.0 |
2.1 |
|||||||
|
Over 40kgs |
Kflow Epic |
14FG X 31cm |
36cm |
31cm |
2.0 |
2.1 |
|||||||
6.1 Follow up
- Children will often be followed up by local teams with renal network support
- A BP and urinalysis with PCR should be checked at each visit and consider creatinine at appropriate intervals.
- It is recommended patients are followed up yearly for a minimum of 5 years
- Children should have a formal GFR study performed at 18 months post discharge.
- A corrected GFR >100 (greater than 100) is considered normal.
- Children with a GFR <100 (less than 100) should be re-discussed with the renal network clinician.
- It may be suitable for the GP to continue follow up at the discretion of the leading clinician.
- Females who have recovered from STEC-HUS should be closely monitored in pregnancy
Haemolytic Uraemic Syndrome may follow infection with other agents, including Strep. Pneumoniae, Klebsiella and Shigella therefore other potential infectious agents should be considered.
Familial cases of HUS (atypical haemolytic uraemic syndrome) are also recognized and a detailed family history should be taken, including the possibility of consanguinity.
All patients should be referred to the on-call paediatric nephrologist where HUS is a prominent clinical feature.
National guidance and details on sample requirements for aHUS can be found at http://www.atypicalhus.co.uk/home/emergency-referrals/
7.1 Other potential causes
- Idiopathic
- Drug Toxicity
- Chemotherapy, Mitomycin-C, Tacrolimus, Cyclosporin, oral contraceptives, valacyclovir, Ticlodipine, Clopidorgel
- Conditioning for Bone Marrow Transplantation
- Solid organ transplantation
- Malignancy
- Hereditary: Inborn error of cobalamin deficiency
- Pregnancy or post partum
- AIDS and early symptomatic HIV
- Connective Tissue Disease: SLE, Sjorgen's, Systemic Sclerosis, antiphospholipid antibody syndrome, Scleroderma
- Other glomerulonephritides e.g. APIGN, membranoproliferative
7.2 Possible Investigations
- Complement levels
- Low C3, Factor H, C3 Nephritic Factor
- Genetics: complement
- Von Willebrand cleaving factor
- Urinary methylmalonic acid
- Homocysteine
- Renal Biopsy
- Autoantibodies
- Anticardiolipin, ANA, Anti ds DNA, Anti ENA
- Antiplatelet antibodies
- Pregnancy Test
- HIV Test
Should any aspect of this guideline change before the planned review then this guideline should be updated accordingly.
Future review of this guideline should make use of the AGREE document to ensure that this guideline incorporates up-to-date evidence and best clinical practice. For further information on guideline development please contact the chairperson of the multiprofessional Clinical Practice Committee.
Please direct any non-urgent queries about the protocol and development to victoria.harkins2@ggc.scot.nhs.uk
Link to Public health E.Coli Information leaflet TBC
- Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, Christie DL, et al. Relative Nephroprotection During Escherichia coli O157:H7 Infections: Association With Intravenous Volume Expansion. Pediatrics [Internet]. 2005 Jun 1 [cited 2018 Oct 4];115(6):e673–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15930195
- Ardissino G, Tel F, Possenti I, Testa S, Consonni D, Paglialonga F, et al. Early Volume Expansion and Outcomes of Hemolytic Uremic Syndrome. Pediatrics [Internet]. 2016 Jan 1 [cited 2018 Aug 6];137(1):e20152153. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26644486
- Hickey CA, Beattie TJ, Cowieson J, Miyashita Y, Strife CF, Frem JC, et al. Early Volume Expansion During Diarrhea and Relative Nephroprotection During Subsequent Hemolytic Uremic Syndrome. Arch Pediatr Adolesc Med [Internet]. 2011 Oct 1 [cited 2018 Sep 7];165(10):884. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21784993
- Mody RK, Gu W, Griffin PM, Jones TF, Rounds J, Shiferaw B, et al. Postdiarrheal Hemolytic Uremic Syndrome in United States Children: Clinical Spectrum and Predictors of In-Hospital Death. J Pediatr [Internet]. 2015 Apr [cited 2018 Nov 11];166(4):1022–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25661408
- Mele C, Remuzzi G, Noris M. Hemolytic uremic syndrome. [cited 2018 Nov 12]; Available from: https://link.springer.com/content/pdf/10.1007%2Fs00281-014-0416x.pdf
- Balestracci A, Martin SM, Toledo I, Alvarado C, Wainsztein RE. Dehydration at admission increased the need for dialysis in hemolytic uremic syndrome children. Pediatr Nephrol [Internet]. 2012 Aug 3 [cited 2018 Nov 14];27(8):1407–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22476204
- Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, et al. Longterm Renal Prognosis of Diarrhea-Associated Hemolytic Uremic Syndrome. JAMA [Internet]. 2003 Sep 10 [cited 2018 Dec 19];290(10):1360. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12966129
- Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term Risk of Mortality and Other Adverse Outcomes After Acute Kidney Injury: A Systematic Review and Metaanalysis. Am J Kidney Dis [Internet]. 2009 Jun [cited 2018 Sep 11];53(6):961–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19346042
- Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, Boster DR, et al. Risk Factors for the Hemolytic Uremic Syndrome in Children Infected With Escherichia coli O157:H7: A Multivariable Analysis. Clin Infect Dis [Internet]. 2012 Jul 1 [cited 2018 Nov 14];55(1):33–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22431799
- Bell BP, Griffin PM, Lozano P, Christie DL, Kobayashi JM, Tarr PI. Predictors of hemolytic uremic syndrome in children during a large outbreak of Escherichia coli O157:H7 infections. Pediatrics [Internet]. 1997 Jul [cited 2018 Dec 20];100(1):E12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9200386
- Trachtman H, Austin C, Lewinski M, Stahl RAK. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol [Internet]. 2012 Nov 18 [cited 2018 Nov 14];8(11):658–69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22986362
- Holtz LR, Neill MA, Tarr PI. Acute Bloody Diarrhea: A Medical Emergency for Patients of All Ages. YGAST [Internet]. 2009 [cited 2019 Feb 26];136:1887–98. Available from: http://www.documents.
- Ardissino G, Daccò V, Testa S, Civitillo CF, Tel F, Possenti I, et al. Hemoconcentration: a major risk factor for neurological involvement in hemolytic uremic syndrome. Pediatr Nephrol [Internet]. 2015 Feb 23 [cited 2019 Feb 22];30(2):345–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25149851
- Grisaru S, Xie J, Samuel S, Hartling L, Tarr PI, Schnadower D, et al. Associations Between Hydration Status, Intravenous Fluid Administration, and Outcomes of Patients Infected With Shiga Toxin–Producing Escherichia coli: A SYstematic Review and Meta-analysis. JAMA Pediatr [Internet]. 2017 Jan 1 [cited 2019 Jun 22];171(1):68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27893870
- Michael M, Elliott EJ, Ridley GF, Hodson EM, Craig JC. Interventions for haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Cochrane Database Syst Rev [Internet]. 2009 Jan 21 [cited 2018 Nov 16];(1):CD003595. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19160220
- Gianviti A, Tozzi AE, De Petris L, Caprioli A, Ravà L, Edefonti A, et al. Risk factors for poor renal prognosis in children with hemolytic uremic syndrome. Pediatr Nephrol [Internet]. 2003 [cited 2018 Nov 22];18:1229–35. Available from: https://link.springer.com/content/pdf/10.1007%2Fs00467-003-1262-6.pdf
- Clinical Guidance on the Assessment of Bloody Diarrhoea and Suspected or Confirmed Shiga toxin-producing Escheria Coli (STEC) Infections in Children and Adults Good Practice Guidance Scottish Health Protection Network 2018
Last reviewed: 01 August 2019
Next review: 01 August 2022
Author(s): David Hughes
Version: 2
Approved By: AWAITING APPROVAL BY: Paediatric Clinical Effectiveness & Risk Committee

