Anaemia in children with chronic kidney disease
Objectives
Guideline objectives:
- Explain why it is important to diagnose and treat anaemia in children with CKD
- Define anaemia in children with CKD
- Outline tests required to investigate the cause of anaemia in children with CKD
- Provide information on the pharmacological management of anaemia
- Provide guidance on ongoing monitoring required for children with anaemia and CKD
Clinical questions answered by the guideline:
- How is anaemia defined in children according to age and gender?
- How is anaemia investigated?
- What therapies are used to treat anaemia?
- What follow-up and monitoring do patients with anaemia require?
Scope
This document provides information on the diagnosis and management of anaemia in children with chronic kidney disease (CKD) throughout Scotland, including those on dialysis or following renal transplantation. In general recommendations are for children with an estimated glomerular filtration rate (eGFR)<60 ml/min/1.73m2 unless stated otherwise. This document is intended for use by all health professionals (for example, doctors, nurses, dieticians and pharmacists) who look after children with CKD within Scotland.
Audience
For use across the Scottish Paediatric Renal and Urology Network
Anaemia is a common feature among children with CKD. Factors contributing to anaemia include:
- Decreased production of erythropoietin
- Iron, vitamin B12, folate and copper deficiency
- Bone marrow inhibition due to inflammation and uraemia
- Regular blood loss (e.g., haemodialysis patients where blood circuit may be lost)
- Decreased red cell survival
- Hyperparathyroidism
- Haemoglobinopathies
- Haemolysis (e.g., due to turbulence within HD circuit or due to an underlying disorder such as atypical HUS)
- Rejection episodes in transplant recipients
- Delayed graft function
- Medications, e.g., immunosuppressant agents (e.g., azathioprine, MMF), angiotensin converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARB), trimethoprim, sulfamethoxazole, calcium channel blockers and valganciclovir.
Anaemia is associated with reduced survival in adult dialysis patients. Adverse effects of anaemia in patients with CKD include reduced oxygen utilisation, increased cardiac output and left ventricular hypertrophy, and impairment of cognitive function and quality of life. Conversely, higher haemoglobin (Hb) values have been associated with improved health-related quality of life, exercise tolerance and a reduction in cardiac index in children on dialysis.
Anaemia is potentially reversible and therefore is important to recognise and treat.
Due to a lack of long-term outcome studies, the optimal haemoglobin (Hb) level for children with CKD is not known. The physiological age dependence of Hb levels adds another level of complexity to paediatric anaemia management. The NKF-KDOQI guidelines define anaemia as a Hb level below the 5th percentile for age and gender in children with CKD. Based on studies of anaemia in adults with CKD, the 2015 UK NICE Anaemia Management in CKD guidelines suggested a target Hb of 100 to 120 g/L, or 95 to 115 g/L if younger than 2 years of age reflecting the lower normal range in that age group (Table 1). Upper limits for target Hb are based on adult studies where a target Hb level 140 ± 10 g/L was associated with higher mortality in patients with cardiovascular disease and was not cost effective. Until outcome studies are performed, children with CKD in Scotland should achieve Hb within the target range defined by the NICE Anaemia Management guideline. However, in a retrospective cohort of paediatric patients on haemodialysis (HD), Hb > 120g/L was not associated with an increased risk cardiovascular morbidity.
|
Age |
Hb (g/L) |
|
< 2 years |
95 – 115 |
|
≥ 2 years |
100 - 120 |
Table 1. Target Hb range for children with CKD in Scotland
Children with CKD whose Hb falls < 110 g/L (or < 105 g/L if younger than 2 years), or those who develop symptoms attributable to anaemia (such as tiredness/lethargy, shortness of breath, and palpitations) should be investigated as to the cause of their anaemia and appropriate therapy instituted thereafter.
Follow the MHRA safety advice on recombinant human erythropoietins, particularly the advice to avoid Hb levels above 120 g/L because of the increased risk of death and serious adverse cardiovascular events in people with CKD. People should have close monitoring to ensure that the lowest approved dose of ESA is used to provide adequate control of the anaemia symptoms. [2021 update]
However, the committee highlighted that coagulation risks in children and young people are very different to those in adults. The committee noted that the current recommended Hb levels may be too low for children as, in practice, higher targets of between 110 -130 g/L are being used, but was unable to draft new recommendations about higher Hb levels because there was no new evidence. The committee agreed that further research in this area was important and highlighted that audit or registry data may also be useful as this would allow data on safety and efficacy to be captured for different Hb targets currently being used in practice. It made a research recommendation to support further research in this area.
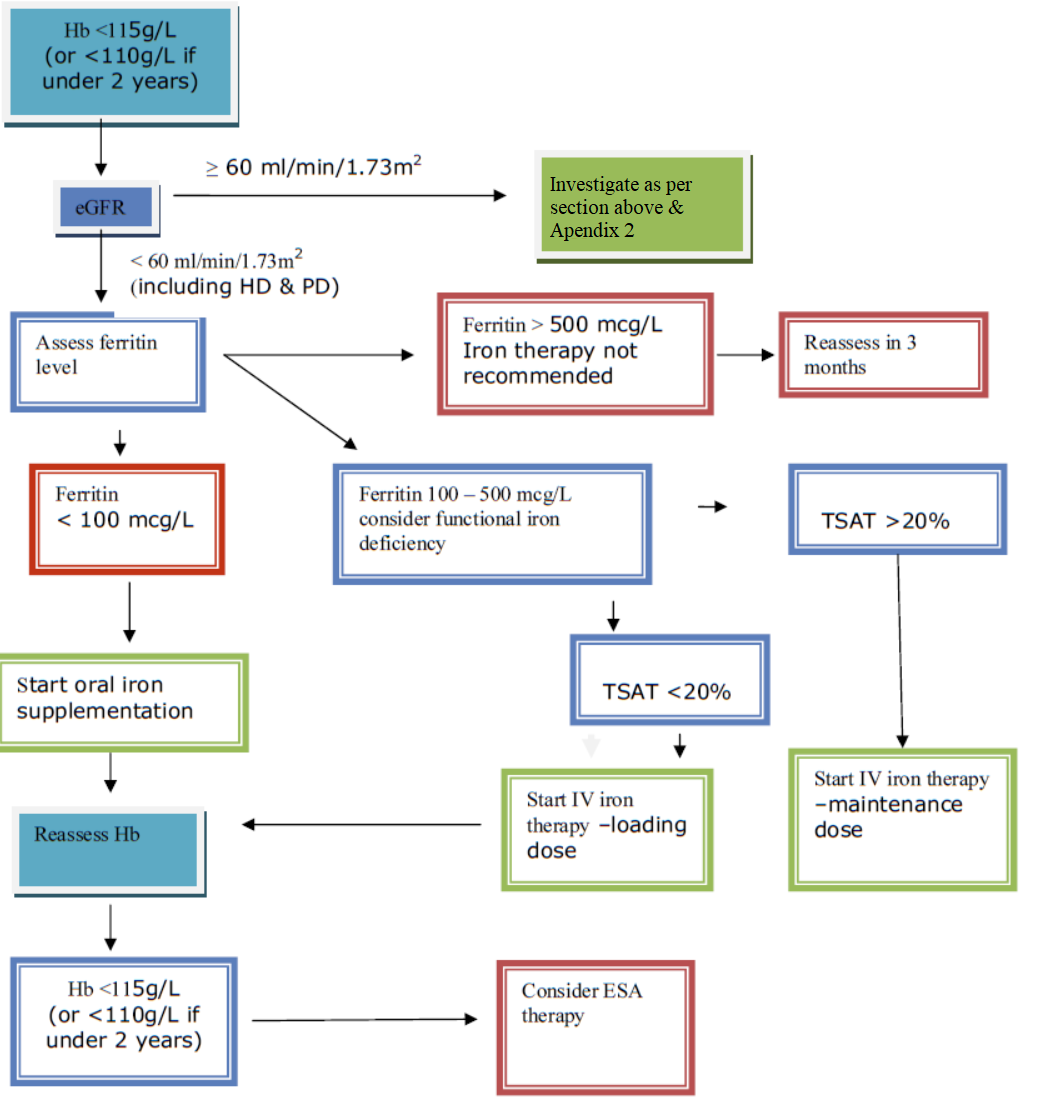
An estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2 should trigger investigation into whether anaemia is due to CKD (Appendix 1). When the eGFR is ≥60 mL/min/1.73m2 the anaemia is more likely to be related to other causes (see below).
Serum ferritin levels and transferrin saturation may be used to assess iron deficiency in people with CKD but it is important not to use ferritin levels alone. Serum ferritin is an acute phase reactant and is frequently raised in CKD. It is therefore useful to consider sending a CRP along with a ferritin level for the purposes of correlation (particularly if the ferritin level is found to be elevated) The diagnostic cut-off value for ferritin can be interpreted differently to non-CKD patients. Iron deficiency anaemia should be diagnosed in children with stage 5 CKD with a ferritin level <100 mcg/L and transferrin saturation <20%, and should be considered in children with stage 3 and 4 CKD if ferritin is <100 mcg/L and transferrin saturation <20%.
Patients with ferritin levels >100 mcg/L may have a functional iron deficiency. These patients are most likely to benefit from intravenous iron therapy. Functional iron deficiency is ideally defined by percentage of hypochromic red cells >6%, or else transferrin saturation <20% (when the measurement of the percentage of hypochromic red cells is unavailable).
Novel markers of iron status, such a reticulocyte haemoglobin content (CHr) and reticulocyte haemoglobin equivalent (Ret-He), have been identified. Where these tests are available, their use is advocated by NICE and European best practice guidelines. These markers have short half-lives, and their synthesis is dependent on iron availability. These are believed to be appropriate markers of iron status and functional iron availability in children with CKD and, with the right equipment, can be analysed using the same EDTA sample used for full blood count (FBC) analysis thereby foregoing the need to send additional blood samples.
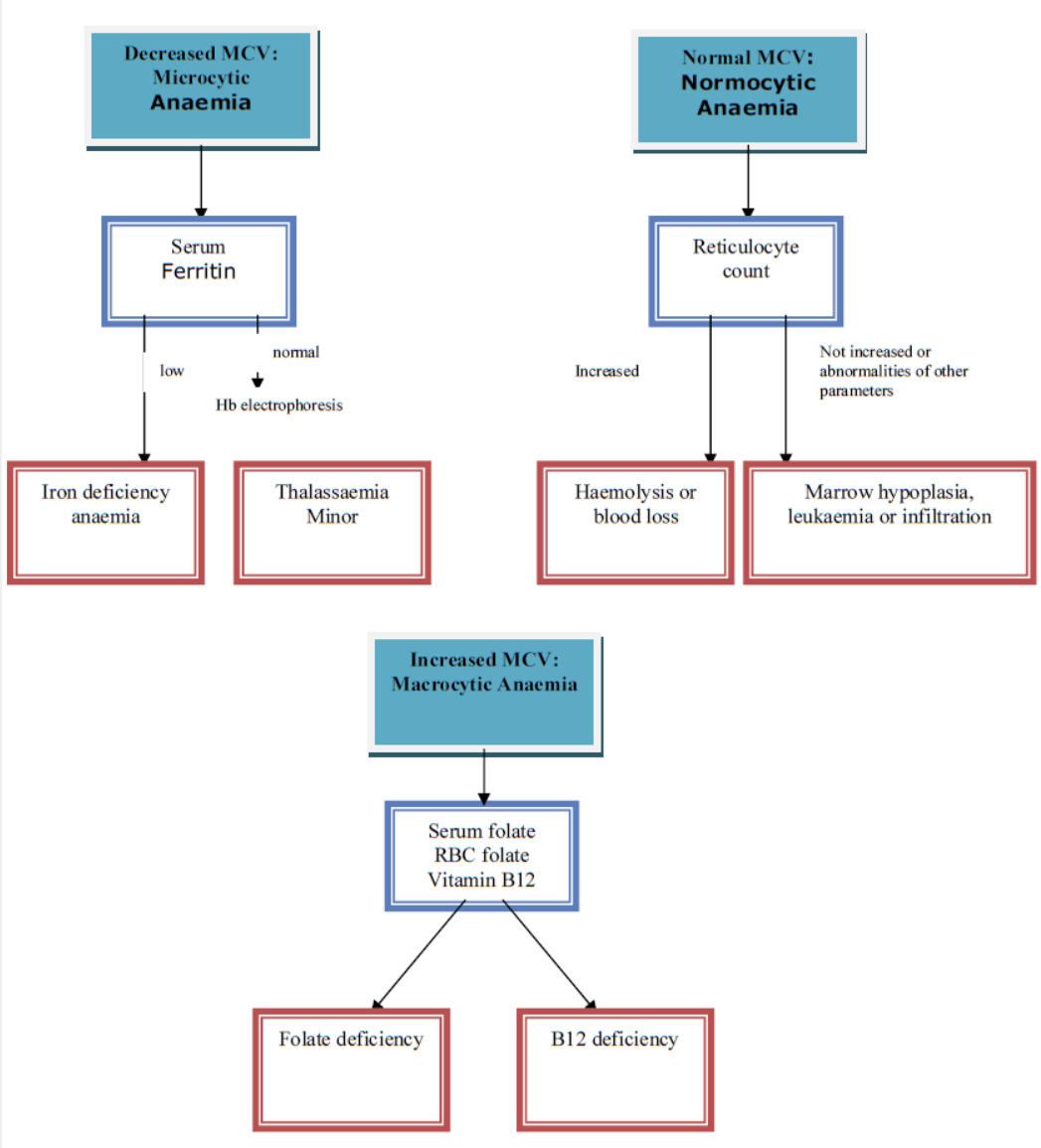
Investigations if eGFR >60 mL/min/1.73m2
Investigations for cause of anaemia in children with eGFR >60 mL/min/1.73m2 are based on the mean cell volume (MCV). Appendix 2 contains a flow diagram which details when the following may be useful:
- FBC and film
- Ferritin, serum folate, red cell folate and vitamin B12
- Haemoglobinopathy screen
In general, anaemia therapy aims to achieve an Hb level consistently within the aspirational target range (Table 1). The pros and cons of a trial of anaemia management should be discussed between the clinician, the patient with anaemia of CKD, and their family/carers. However, when determining individualised aspirational Hb ranges for children and young people with anaemia of CKD, consider the following:
- patient / carer preferences
- symptoms and co-morbidities
- the required treatment
Iron supplementation
Patients with anaemia and a ferritin level <100 mcg/L have iron deficiency anaemia, and will benefit from iron supplementation. Oral iron supplements should be trialled initially. Ferritin and Hb levels should be reassessed after starting iron therapy, once every month to three months depending on the severity of the anaemia and CKD stage, and HD.
Oral iron supplements
There are several oral iron formulations, with differing side effect profiles. In cases where one agent is not tolerated, another should be tried.
The oral dose of elemental iron to treat iron-deficiency anaemia is 3-6 mg/kg (max 200 mg) daily given in 2-3 divided doses. There are a number of different salts of iron available. The dose is calculated by the elemental iron content. Gastrointestinal irritation can occur with iron salts. If side effects do occur, the dose may be reduced or another iron salt may be better tolerated. For up-to-date dosing advice and available oral iron preparations, please refer to the BNF for children (https://bnfc.nice.org.uk).
In children treated with iron, serum ferritin levels should not rise above 800 mcg/L. In order to prevent this, the dose of iron should be reviewed when serum ferritin levels reach 500 mcg/L.
Some patients may not tolerate oral iron supplements due to side effects, in which case IV iron should be considered.
Oral iron supplements should be separated from phosphate binders and food (ideally 2 h before and 1 h after). Proton pump inhibitors (e.g., omeprazole) will reduce iron absorption and therefore should not be given concurrently.
Intravenous iron
Ferritin is an acute phase reactant and may be raised due to chronic inflammation even in patients with iron deficiency. Furthermore, patients with ferritin levels between 100 and 500 mcg/L may have a functional iron deficiency, which is indicated by percentage hypochromic red cells > 6% or transferrin saturation < 20%. Do not check iron levels earlier than 1 week after receiving IV iron. It is also important to ensure that the management of renal bone disease is optimised as patients with secondary hyperparathyroidism are at risk of anaemia. Potential mechanisms include a direct effect of PTH on bone marrow erythroid progenitor cells and on red cell survival through accelerated haemolysis, and an indirect effect through induction of bone marrow fibrosis.
Venofer® (iron (III)-hydroxide sucrose complex) is the most commonly used IV iron preparation in paediatrics. It must not be administered by SC or IM route. Secure IV access must be obtained prior to administration as extravasation with Venofer® causes a painful tissue reaction. Hypotension may occur if the injection is administered too rapidly.
Ferinject® (ferric carboxymaltose) is a new IV iron preparation which is licensed in children over 14 years of age, although it has been given safely and effectively to children with iron deficiency anaemia from 9 months to 18 years. It must not be administered by SC or IM route. Secure IV access must be obtained prior to administration. It is given as a single large dose with monitoring of haematinics 4 weeks post dose, and further doses are given when necessary.
- IV iron products should only be administered where there is immediate access to resuscitation facilities and staff trained to evaluate and manage anaphylactoid or anaphylactic reactions.
- Patients should be closely monitored for signs of hypersensitivity during, and for at least 30 minutes after, every administration of an IV iron product.
All loading doses of Venofer® can be administered as an IV infusion within the clinic setting. Further maintenance doses of Venofer® can be administered as a slow bolus injection.
See Appendix 3 to calculate Venofer® loading and maintenance doses.
See Appendix 4 to calculate Ferinject® doses.
Erythropoiesis-stimulating agents (ESAs)
The advent of recombinant human erythropoietin (EPO) in the late 1980s resulted in a dramatic reduction in the number of blood transfusions used in dialysis centres. The risk of HLA sensitisation from blood transfusions means that they should be avoided in patients with CKD whenever possible.
Plasma EPO has a fairly short circulating half-life (approximately 6 to 8 h) so patients may require two or three injections a week. Longer acting ESAs such as darbepoetin alfa or Continuous EPO Receptor Activator (CERA), are protein-based, bearing some structural resemblance to EPO itself. Modifications have been made to the EPO molecule to allow it to have a longer duration of action in vivo. Protein-based therapies have a number of disadvantages, notably immunogenicity (pure red cell aplasia caused by anti-EPO antibodies), storage and stability (must be stored at temperatures of approximately 4°C), and administration (all currently licensed products are administered via IV or SC routes).
Note: ESAs need not be administered where the presence of co-morbidities, or the prognosis, is likely to negate the benefits of correcting the anaemia. Where a trial of ESA therapy has been performed, the effectiveness of the trial should be assessed after an agreed interval. Where appropriate, a mutual decision should be agreed between the clinician, the child and their family/carer on whether or not to continue ESA therapy.
Correction to normal levels of Hb with ESAs is not usually recommended in people with anaemia of CKD.
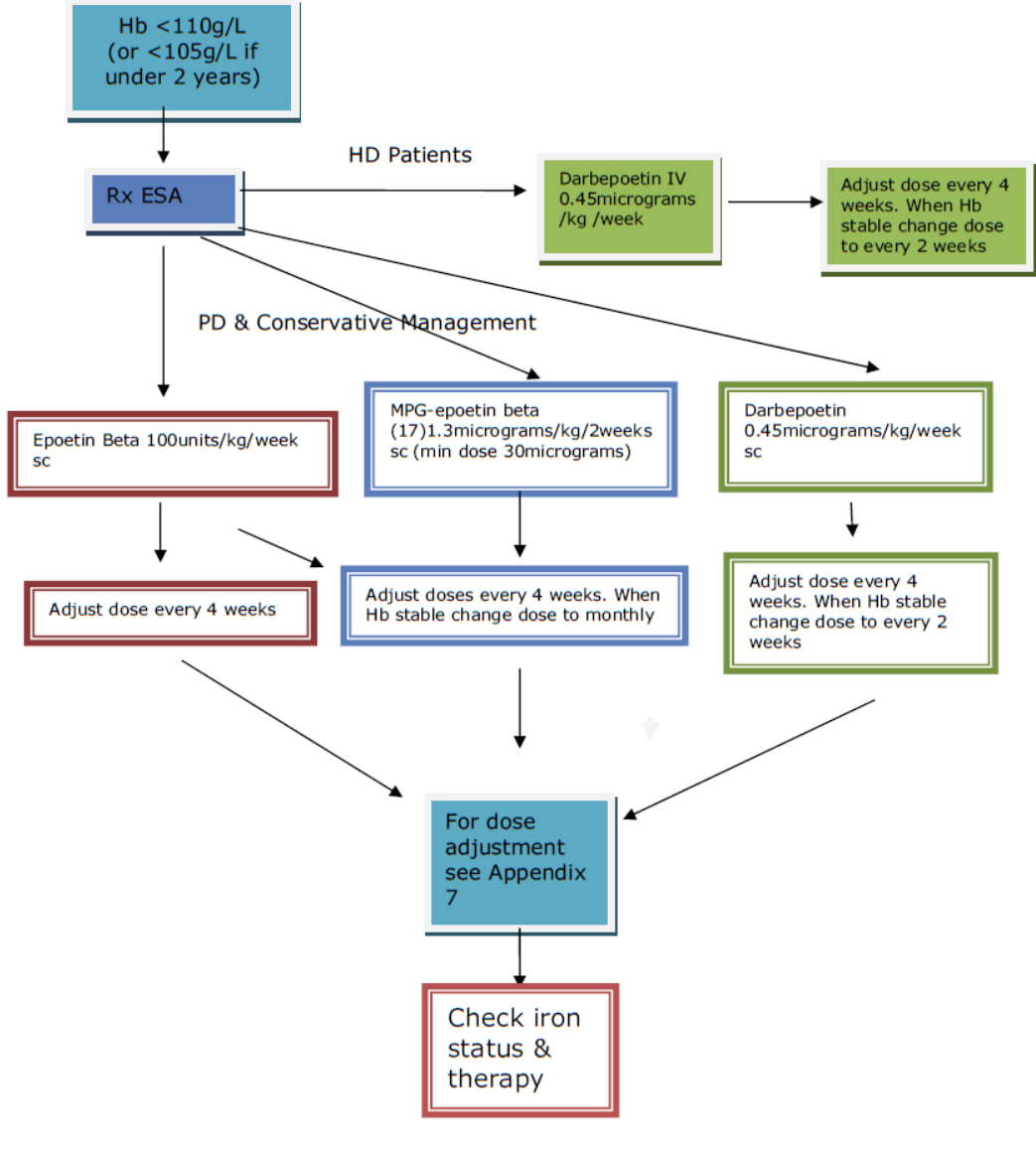
Epoetin beta (NeoRecormon®)
Epoetin beta is first choice ESA in younger children under 12kg or less than 12 months of age attending the renal unit who are on peritoneal dialysis (PD) or receiving conservative management. It is normally given by SC injection (can be given by IV injection but is less effective if given in this manner). For starting dose see Appendix 5.
Darbepoetin alfa (Aranesp®)
Darbepoetin is a hyperglycosylated derivative of EPO with a longer half-life. It is the first choice for children commencing HD. It is given IV during HD initially once a week then changed to every 2 weeks once established on a stable dose. For starting dose see Appendix 5. Darbepoetin can also be given by SC injection to children attending the renal unit who are on PD or receiving conservative management. However, pain at the site of injection is more common than in children receiving epoetin beta. For this reason, it is a third choice here (behind epoetin beta and Mircera [see below]). However, it has the advantage that it can be given less frequently than epoetin beta.
Methoxy polyethylene glycol-epoetin beta (Mircera®)
Methoxy polyethylene glycol (MPG)-epoetin beta is a newer long-acting ESA. There is very little dosing and safety information available for its use in paediatrics. It is administered SC and has the advantage of being able to be administered every month. It is the second line choice for children on PD or receiving conservative management. For dose information see Appendix 5.
Conversion doses for ESA therapy
There are circumstances when children on one form of ESA therapy will require a change to another. Conversion doses can be calculated using the information in Appendix 6: Table 3. Doses may need to be rounded up or down due to the strengths available and frequency changed to weekly for epoetin beta, every 2 weeks for darbepoetin, and monthly for MPG-epoetin.
Adjusting ESA therapy
After commencing ESA therapy, the frequency at which the Hb level is rechecked will be determined by which ESA agent is used. In general, Hb should be monitored:
- every 2–4 weeks in the induction phase of ESA therapy
- every 1–3 months in the maintenance phase of ESA therapy
- more actively after any ESA dose adjustment
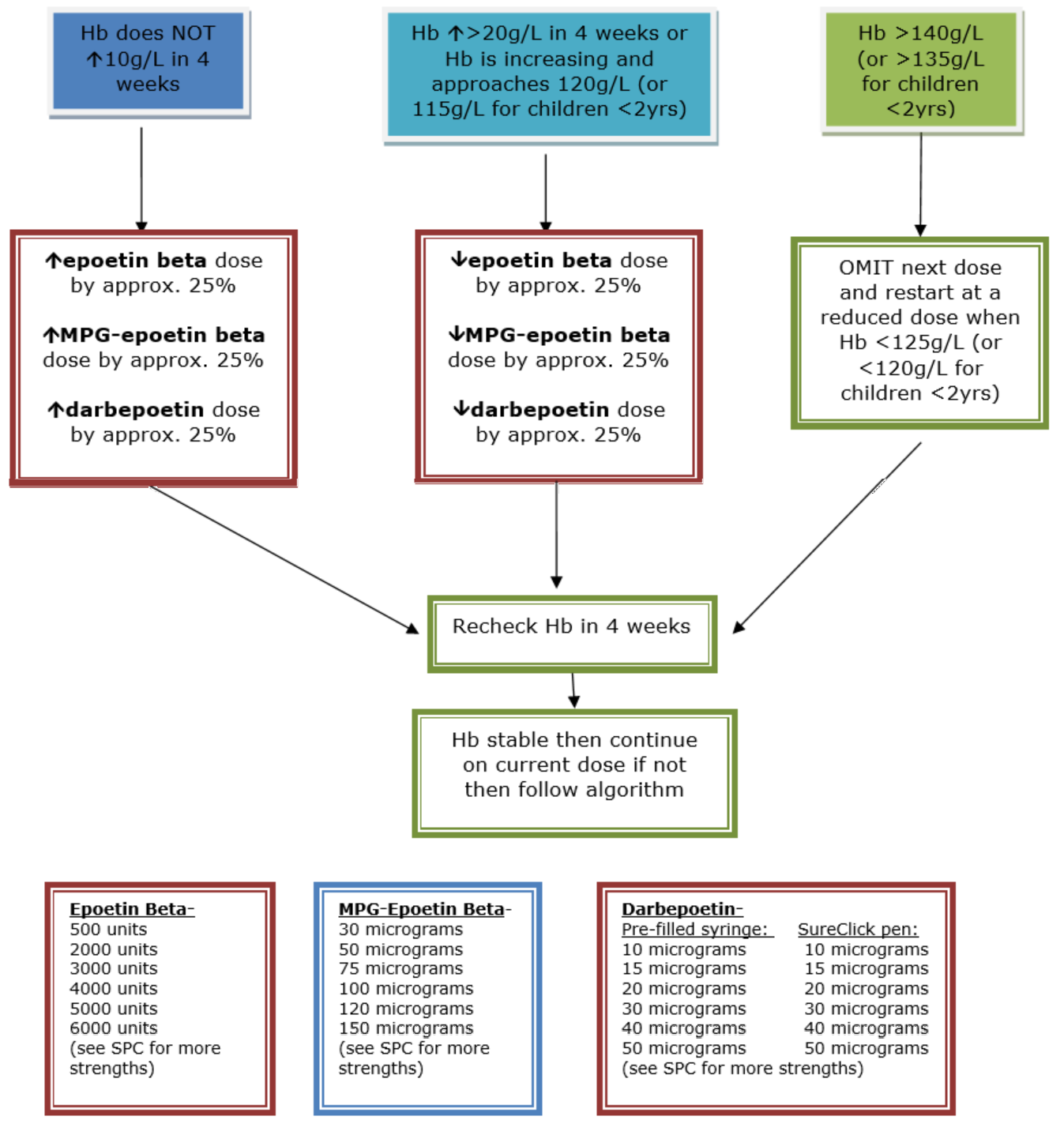
ESA therapy dose should be adjusted as needed to maintain the Hb level within the aspirational target range (see Appendix 7 for dose adjustment algorithms). To keep the Hb level within the aspirational range, action should be taken when Hb levels are within 5 g/L of the range’s limits (usually < 105 g/L or > 115 g/L for children over 2 years), or if the rate of change of Hb suggests an established trend (for example, > 10 g/L/month).
Consider accepting lower Hb levels if:
- High doses of ESAs are required to achieve the aspirational range
> 175 IU/kg/week equivalent Epoetin for HD population;
> 125 IU/kg/week equivalent Epoetin for PD population;
> 100 IU/kg/week equivalent Epoetin for non-dialysis population; or
- The aspirational range is not achieved despite escalating ESA doses.
Consider accepting Hb levels above the agreed aspirational range when:
- These develop with iron therapy alone, or
- These develop with low doses of ESAs, or
- It is thought that the person might benefit (for example, children who are athletic and very active), or
- The absolute risk of cerebrovascular disease is thought to be low.
An unexpected change in Hb level should be investigated to enable intervention and optimise iron status. Causes of a change in Hb level include intercurrent illness, bleeding, and the addition of new medications (for example immunosuppressant agents).
The use of ACEI or ARBs is not precluded, but increased ESA therapy may be required if they are used.
Detecting and managing ESA resistance
Non-adherence to ESA (and/or iron) therapy must first be excluded in the patient who appears not to respond to ESA therapy. Where non-adherence is suspected, further action could include checking whether the ESA is being prescribed by the GP and whether the medication is being collected from the community pharmacy. Where non-adherence is suspected, or if children find the injections painful such that parents may not be successfully administering the full dose, the ESA may need to be administered at the hospital clinic, by the GP practice nurse, or by the community children’s nursing team.
Having excluded non-adherence, the following should be excluded:
- Intercurrent illness (e.g., parvovirus infection)
- Chronic blood loss
Consider GI investigations - Haemolysis (check blood film for fragments, LDH, and haptoglobin)
- Aluminium toxicity
This is unlikely, due to rare use of aluminium-containing phosphate binders - Nutritional factors (e.g. B12, folate and copper deficiency)
- Medications (mentioned in Section on ESAs)
- Poorly-controlled secondary hyperparathyroidism
Patients should be considered resistant to ESAs when:
- The aspirational Hb range is not achieved despite treatment with:
- ≥ 300 IU/kg/week subcutaneous epoetin beta
- ≥ 450 IU/kg/week intravenous epoetin beta
- >1.5 micrograms/kg/week darbepoetin alfa
- > 4 micrograms /kg/every 2 weeks MPG-epoetin (there is no max dose stated in the SPC this dose is calculated on the equivalent dose of epoetin beta)
- Continued high doses of ESAs are needed to maintain Hb within the aspirational Hb range
Consider referring children with ESA resistance to the haematology service particularly if an underlying haematological disorder is suspected.
Review the frequency of needing red cell transfusions and consider a trial period of stopping ESA in patients who have ESA resistance (typically on HD and on high-dose ESA) if:
- All reversible causes of ESA resistance have been taken into account and excluded
- The child’s condition is otherwise stable
- The child is receiving adequate dialysis
Review the frequency of needing red cell transfusions between 1-3 months after stopping ESA therapy. If this has increased, then consider restarting an ESA.
Rarely, patients may become ESA resistant due to the formation of anti-erythropoietin antibodies. This is known as pure red cell aplasia (PRCA) and is indicated by:
- low reticulocyte count
- anaemia
- anti-erythropoietin antibodies
PRCA should be confirmed by the presence of anti-erythropoietin antibodies in the serum together with a lack of pro-erythroid progenitor cells in bone marrow.
Frequency of Monitoring for patients on ESA therapy
- Monitoring of haematological parameters (Hb, ferritin and transferrin saturation) should be performed monthly, or at each clinic visit if less often than monthly
Standards for laboratory and clinical indices:
- Hb should be maintained within the individualised aspirational target range
- Ferritin should be maintained between 100 and 500 mcg/L
- Serum PTH levels should be maintained at less than twice the upper limit of normal.
Roxadustat (Evrenzo®) is a new orally licensed medication (August 2021) for the management of symptomatic anaemia associated with CKD in adults not currently treated with an ESA. It is a hypoxia-inducible factor, prolyl hydroxylase inhibitor (HIF-PHI), which stimulates a coordinated erythropoietic response, thereby increasing Hb production and improving iron bioavailability. A phase 3 clinical trial in paediatric patients with CKD is ongoing.
Calculation of Correction Dose:
- Total Iron Deficit = in mg
- {wt(kg) x [(target Hb-actual Hb)(g/L) x 0.24]} + depot iron requirements
- Depot iron requirements = in mg
- Weight <35 kg: 15 mg/kg (max 500 mg)
- Weight >35 kg: 500 mg
- Round up Total Iron Deficit to nearest 10 mg
- Administer the correction dose in divided amounts
- Each divided dose should not exceed 3 mg/kg/dose or 200 mg/dose whichever is the smallest
- Administer max 3 loading doses per week (this may be done on 3 consecutive days) by infusion
- Dilute 100 mg Venofer® in 100 mL of sodium chloride 0.9% (concentration 1 mg/ml) and use immediately
- Administer dose at a rate not greater than 3 mL/kg/hour.
IV iron and serious hypersensitivity reactions:
- IV iron products should only be administered where there is immediate access to resuscitation facilities and staff trained to evaluate and manage anaphylactic or anaphylactoid reactions.
- Patients should be closely monitored for signs of hypersensitivity during, and for at least 30 minutes after every administration of an IV iron product.
Maintenance Dose:
- Weight <50 kg: 2 mg/kg, corrected to nearest 5 mg, every 2-4 weeks as single IV dose
- Weight >50 kg: 100 mg as a single IV dose every 2-4 weeks
- If ferritin >500 mcg/L then omit dose until ferritin <500 mcg/L
Calculation of Dose:
A single Ferinject administration should not exceed:
- 15 mg iron/kg body weight
|
Ferritin (micrograms/L) |
Transferrin Saturation (%) |
Weight |
Ferric carboxymaltose (Ferinject) dose |
Concentation & Rate of infusion |
|
<100 |
<20 |
35-50 kg |
500 mg |
500 mg in 50 mL run over 30 mins |
|
<100 |
<20 |
51-66 kg |
750 mg |
750 mg in 75 mL run over 30 mins |
|
<100 |
<20 |
>66 kg |
1000 mg |
1000 mg in 100 mL run over 30 mins |
|
Ferritin (micrograms/L) |
Transferrin Saturation (%) |
Weight |
Ferric carboxymaltose (Ferinject) dose |
Concentation & Rate of infusion |
|
100 -500 |
<20 |
35-50 kg |
500 mg |
500 mg in 50 mL run over 30 mins |
|
100 -500 |
<20 |
51-66 kg |
500 mg |
500 mg in 50 mL run over 30 mins |
|
100 -500 |
<20 |
>66 kg |
500 mg |
500 mg in 50 mL run over 30 mins |
For infusion, Ferinject must only be diluted in sterile 0.9% sodium chloride solution. Note: for stability reasons, Ferinject should not be diluted to concentrations less than 2 mg iron/mL (not including the volume of the ferric carboxymaltose solution).
Max dose per week 1000mg.
Warning: The risk of persistent hypophosphataemia and osteomalacia may be higher with ferric carboxymaltose (Ferinject®) than with other intravenous iron formulations. A key mechanism postulated is that the carbohydrate moieties in ferric carboxymaltose (Ferinject®) may disproportionately inhibit degradation of fibroblast growth factor 23 (FGF23), which can result in increased FGF23 activity and ultimately greater renal phosphate wasting.
IV iron and serious hypersensitivity reactions:
- IV iron products should only be administered where there is immediate access to resuscitation facilities and staff trained to evaluate and manage anaphylactic or anaphylactoid reactions.
- Patients should be closely monitored for signs of hypersensitivity during, and for at least 30 minutes after every administration of an IV iron product.
(NB: Doses may need to be rounded up or down due to the strengths available and frequency changed to weekly for Epoetin beta, every 2 weeks for darbepoetin and monthly for MPG-epoetin.)
|
ESA Conversion |
Calculation |
Example |
|
Epoetin beta to Darbepoetin |
Epoetin beta(units/week) |
2400units/week of Epoetin beta = |
|
Epoetin beta to |
100units/kg/week epoetin beta = 2.6micrograms/kg/monthly |
50kg child on 5000units/week of |
|
Darbepoetin to |
Darbepoetin |
20micrograms/week of Darbepoetin |
|
Darbepoetin to |
Darbepoetin |
40micrograms/month of Darbepoetin = |
|
MPG-epoetin to |
2.6micrograms/kg/monthly |
30kg child on 75micrograms/month of MPG-epoetin = 3000units/week of Epoetin beta |
|
MPG-epoetin to |
MPG-epoetin |
100micrograms/month MPG-epoetin |
Table 3. Conversion doses for ESA therapy.
- Rees L et al Paediatric Nephrology 3rd Edition Oxford: Oxford University Press; 2019
- Renal Association Clinical Practice Guideline, 2017. Anaemia of Chronic Kidney Disease.
- Plumb L, Casula A, Magadi W et al. UK Renal Registry 20th Annual Report (July 2018): Chapter 11 Haematological and biochemical parameters in patients on renal replacement therapy in Paediatric Centres in the UK in 2016: National and Centre-specific analyses. Nephron 2018;139 (Suppl 1):c273-286.
- National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Section III. Clinical practice recommendations fro anaemia in chronic kidney disease in children. American Journal of Kidney Disease 2006 (Suppl 3) 47:S86-108.
- The National Clinical Guideline Centre. NICE guidelines - Chronic kidney disease: anaemia management. 3 June 2015
- Locatelli F, Aljama P, Bárány P, et al. European Best Practice Guidelines Working Group. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004 May;19 Suppl 2:ii1-47
- Chronic kidney disease [J] Evidence reviews for aspirational haemoglobin target range for children and young people with CKD NICE guideline NG203 Evidence reviews underpinning recommendations 1.9.11 and research recommendations in the NICE guideline August 2021
- Rheault MN, Molony JT, Nevins T et al. 2017. Hemoglobin of 12g/dl and above is not associated with increased cardiovascular morbidity in children on hemodialysis. Kidney International 91 (1):177-182
- BNF for Children (BNFc)
- BNF
- EMC Ferinject
- EMC Evrenzo®
- Powers JM, Shamoun M, McCavit TL, Adix L, Buchanan GR: Intravenous Ferric Carboxymaltose in Children with Iron Deficiency Anemia Who Respond Poorly to Oral Iron. Journal of Pediatrics 180 (pp212-216) 2017
- Warady BA, Barcia J, Benador N et al. 2018. De novo weekly and biweekly darbepoetin alfa dosing in pediatric patients with chronic kidney disease. Pediatric Nephrology 33(1): 125-137.
- Schaefer F, Hoppe B, Jungraithmayr T, et al. 2016. Safety and usage of darbepoetin alfa in children with chronic kidney disease prospective registry study. Pediatric Nephrology. 31(3): 44353.
- Wedekin M, Ehrich JHH, Pape L. Effective treatment of anaemia in paediatric kidney transplant recipients with methoxy polyethylene glycol-epoetin beta. Pediatric Transplantation 2011;15:329-33
- Cano F, Alarcon C, Azocar M, Lizama C, Lillo AM, Delucchi A, Gonzalez M, Arellano P, Delgado I and Droguett MT. Continuous EPO receptor activator therapy of anaemia in children under Peritoneal Dialysis. Paediatric Nephrology 2011 ;26:1303-
- Bruce G, Schulga P, Reynolds BC. Use of erythropoiesis-stimulating agents in children with chronic kidney disease: a systematic review. Clinical Kidney Journal 2022; sfac058.
Last reviewed: 12 April 2022
Next review: 12 April 2026
Author(s): Angela Lamb
Version: 1.3
Approved By: Renal Clinicians Group

