- Good infection control practice is of paramount importance in reducing the spread of infection. Always clean your hands before and after each patient contact. Alcohol gel is effective, although if hands are visibly soiled please use soap and water
- The epidemiology of neonatal sepsis varies geographically and is constantly changing, with secular trends being interrupted by outbreaks and epidemics.
- Good antimicrobial stewardship and avoidance of inappropriate antimicrobial use, can reduce morbidity and mortality. Regular multidisciplinary discussions involving local antimicrobial management teams, microbiologists and infection control colleagues are recommended to inform and review practices. Surveillance systems that enable regular review of local epidemiology are an integral part of this process.
- Infection prevention and control teams should have systems and triggers in place whereby problem assessment group or incident management group meetings are initiated in a timely manner where appropriate.
- Sepsis in neonates can be rapidly aggressive, and is associated with later complications. (1)(2)
- Liaison with a microbiologist familiar with neonatal practice is recommended for atypical organisms or poor clinical response.
- In some parts of the world antibiotic resistance is widespread among the commonest organisms seen infecting neonates.(3)(4)(5)(6-8)(9) This is likely to be a result of national as well as local antibiotic prescribing patterns.
- MRSA, vancomycin-resistant enterococci, gentamicin-resistant coliforms, extended-spectrum beta-lactamase producing coliforms, and carbapenemase-producing organisms are among some of the important organisms to consider when performing screening swabs and making antimicrobial decisions. There may, infrequently, be instances where it is considered necessary to take maternal microbiology results into account when treating neonatal infections. Infection prevention and control guidelines should be consulted and early discussions initiated with local microbiology and infection control teams in any situation where there is uncertainty.
- Given that the Cochrane systematic reviews found only 2 small studies comparing different antibiotic regimens in early onset sepsis, and only 1 small study in late onset sepsis, clearly there is a need for more evidence on which to base guidelines such as this one.(10)(11)(12)
- Infection control is crucial for prevention of nosocomial infection, and policies on breast milk feeding and venous catheters will also impact on infection rates.(13)(14)(15)(16)
- The long term consequences of abnormal gut colonization due to antibiotic pressure, which can be persistent, are unclear.(17)
- Antibiotics should be reviewed daily. A 36 hour review (Early onset sepsis - EOS) or 48h (Late onset sepsis - LOS) is recommended as a decision point at which to document the need for ongoing antimicrobial therapy. Thereafter, antibiotics should be reviewed daily.
[NICE NG195; 2021. Neonatal infection: antibiotics for prevention and treatment] - The antimicrobial suggestions in this document are guidelines only. Each circumstance is different and previous microbiology results, knowledge of local hospital epidemiology and level of acute clinical concern may dictate choice of antimicrobial therapy in any given situation.
Antibiotic guidelines for the neonatal unit

Objectives
This guideline is applicable to medical, nursing and midwifery staff working with neonates in West of Scotland. Prevention of sepsis is important and all staff should be familiar with other guidelines relating to hand hygiene and aseptic technique. Staff should also refer to West of Scotland Neonatal MCN guideline for Early Onset Sepsis in the Neonate, and to the relevant pharmacy monographs for medications referred to in this guideline. It is important to remember to liaise closely with microbiology and infection control departments. This guideline is intended as a support tool and does not replace or over-ride clinical decisions with regards antimicrobial choices in individual scenarios. Junior doctors should always remember to seek senior clinical input for severely unwell patients.
Group B Streptococcus – see the West of Scotland Early onset Sepsis guideline.
Streptococcus pneumoniae - Maternal vaginal colonization with S. pneumoniae is rare (0.11%), but associated with a high risk of infection in the newborn. Intrapartum prophylaxis with benzylpenicillin as per Group B Streptococcus should be considered.(18)
Group A, C and G Streptococcus – Antibiotics should be administered to mother and baby if either develops suspected or confirmed invasive infection in the neonatal period. Women colonized or infected with these organisms should be treated and have this documented in the casenotes.
Guidelines for prevention and control of group A Streptococci – Journal of Infection (2012)
- FBC
- CRP
- Coagulation – only if low platelets or abnormal bruising suggestive of disseminated intravascular coagulation
- Blood culture
- Cultures should be taken prior to the commencement of antibiotic therapy
- At least 0.5ml should be taken.(19, 20) and ideally 1ml.(21) Bigger samples are more sensitive and are also associated with lower contamination rates.(22) A single sample of at least 1ml is virtually as good as multiple samples (23)
- Using appropriate sterilization techniques, a sample of blood from the umbilical cord can be used for culture purposes. This may help address the issue with small volume samples.
- If colonization of a surgically implanted central venous line is strongly suspected, a separate sample may be taken from the central line in addition to the peripheral sample. (This is not usually possible or necessary for PICC Lines).
- Urine culture - If a urinary source for sepsis is suspected (late onset sepsis only), urine should be obtained by suprapubic aspiration. Manual urine microscopy is undertaken in neonates while this is not the practice in older children and adults. This may require a telephone discussion with the on-call biomedical scientist covering microbiology if occurring outside normal working hours or on weekends and public holidays. Also Request specific microscopy if concern about fungal sepsis.
- Lumbar puncture – Lumbar puncture is recommended in any neonate with strongly suspected or proven sepsis (NICE 2021). It is particularly relevant where gram negative or fungal sepsis is suspected.(25)
- Swabs –
- If a surgically placed central line is present, swabs from the exit site may be helpful, if inflammation is present, as they may predict catheter related infection.(26) It is not required to swab the entry site of a PICC line as removal of the dressing risks dislodgement or contamination of the line.
- Surface swabs if infected skin lesion
- Placental swab if chorioamnionitis
- Viral tests – Appropriate to presenting features - see WoS Guideline for Virological assessment of neonates and WoS guidance for management of HSV
- Molecular investigations – 16 or 18s PCR, multiplex PCR, or local point of care tests may be indicated to aid ongoing clinical management. Where local policies and protocols do not exist for this, an early discussion should be had with local microbiology teams as this can oftentimes be performed on culture-negative specimens provided enough sample has been received.
Definition – Infection presenting within the first 72 hours of life. Infecting organism acquired antenatally or intrapartum. May be associated with chorioamnionitis.
Epidemiology
The commonest organisms are from the vaginal flora
- Group B Streptococcus – incidence may be modified by Intrapartum Prophylaxis
- Gram negative bacilli – these include E.coli, Klebsiella species and several others
Less commonly seen
- Staphylococci
- Streptococci – pneumococci, enterococci
- Haemophilus
- Listeria
Empiric therapy
- IV Benzylpenicillin and Gentamicin
Empirical use of Cefotaxime should be avoided on the neonatal unit except in the management of meningitis (see below) as it is associated with higher rates of colonization with resistant strains, Extended Spectrum Beta Lactamase (ESBL) infection, invasive candidiasis and death. (27)(28)(29)
Other conditions to consider
- Bacterial meningitis – IV Cefotaxime and IV Gentamicin
Use Cefotaxime in place of Benzylpenicillin in any of the following cases- CSF analysis demonstrates a leukocytosis
- organisms are found on Gram stain or CSF culture (or PCR when available)
- clinical findings are strongly suggestive of meningitis
NB – If the infant is significantly unwell, and there are no other indicators of the causative organism, consider the addition of Amoxicillin to the antibiotic regime, to cover for Listeria
- Listeria – IV Amoxicillin and Gentamicin
Listeriosis should be considered in the septicaemic or meningitic preterm infant born to a febrile mother with frank chorioamnionitis. A granulomatous skin rash in the newborn would be another indicator. - Herpes Simple Virus (HSV) - Aciclovir. Consider if any of the following are present:
- Thrombocytopenia or coagulopathy
- Hepatomegaly or hepatitis
- Meningitis/encephalitis & seizures
- Unexplained illness in the mother
- Vesicular rash
- See WoS Guideline for the Management of HSV
- Congenital Cytomegalovirus (CMV) – Ganciclovir. Consider if any of the following are present:
- Purpuric ‘blueberry muffin’ rash
- Liver dysfunction
- Colitis
- Respiratory deterioration
- Thrombocytopenia
- See WoS Guideline for the management of CMV
Rationalization
Once an organism has been isolated, the antibiotic should be rationalized to the narrowest spectrum that effectively covers the organism in the site it has been isolated from. See below for specific organisms.
Duration - Consider stopping antibiotics:-
- Where symptoms have resolved - After 2 normal CRP samples at least 18 hours apart.
- Where symptoms persist – After 2 normal CRP samples at least 18 hours apart and blood culture is negative at 36 hours – Consider non-microbiological cause of the symptoms (30)(31)
- After completing minimum duration of treatment for a specific organism identified on blood culture, assuming satisfactory clinical response and normalization of CRP
– refer to the table below for individual organisms
Consider repeat cultures and utilizing antibiotics for late-onset sepsis if there are ongoing signs/symptoms or rising CRP despite 24-48 hours of treatment.
Alert Obstetric Colleagues if blood cultures are positive as the mum may also require treatment.
Definition: Infection presenting after 72 hours of life. Includes nosocomial infections and translocation of organisms across the immature gut wall. Associated with indwelling lines, catheters, ET tubes and drains
Epidemiology - The commonest organisms
- Coagulase negative staphylococci – St. Epidermidis
- Coagulase positive staphylococci – St. Aureus
- Gram negative rods
- Candida
Empiric Therapy
- IV Vancomycin and Gentamicin
Other conditions to consider
- Bacterial meningitis
Add IV Cefotaxime - If clinical findings are suggestive of meningitis
The possibility of meningitis should be considered in those infants with cultures positive for Gram-negative organisms as this will influence the type and length of therapy.
NB – If the infant is significantly unwell, and there are no other indicators of the causative organism, consider the addition of Amoxicillin to the antibiotic regime, to cover for Listeria, especially if the baby has been readmitted from the community
- Fulminant Sepsis OR proven Gram -ve sepsis
Add IV Meropenem
Meropenem is preferential to Piperacillin / Tazobactam (Tazocin) due to better CNS penetration. Tazocin may also be considered in patients taking into account local epidemiology of receiving or referring units, or in selected patients based on clinical judgement.
- Staph Aureus Septicemia and pneumonia in premature infants
Deterioration in premature infants may be associated with fulminant Staph Aureus infection particularly where there is a respiratory deterioration with CXR signs of pneumonia or bullae. In this case surveillance secretions may be positive for Staph Aureus. When suspected, early addition of IV Flucloxacillin to 1st line therapy may be indicated with subsequent rationalization of antibiotics dependent on culture results, clinical response and Xray signs in discussion with microbiology.
- Viral Infection – If symptoms & signs are suggestive of meningitis / encephalitis or there is evidence of liver dysfunction/ coagulopathy, add Aciclovir - For more details please see WoS guidelines for HSV infection and for Virological investigations in the neonate
- Fungal infections – Including yeasts such as Candida species, and moulds.
Consider if Cultures are positive for fungi/moulds or where signs or symptoms of sepsis persist despite appropriate antibiotic therapy as outlined above,
Features which may indicate an increased risk of fungal infection include:-- VLBW babies
- Thrombocytopenia
- Babies who have been on broad-spectrum or prolonged antibiotic courses.
- Where there is known to be candidal colonization of multiple sites or central line sites (32)(33)
Add IV Ambisome - discuss with microbiology. Please note that the liposomal formulation of Amphotericin B will not adequately treat fungal infection of the urinary tract, special formulations of antifungals may be required and these should be discussed with microbiology.
Additional Investigations for fungal infection - ECHO, ophthalmology, chest and abdominal/renal tract imaging is recommended to rule out deep sources
Babies Readmitted From Home
These babies should be managed as per the Paediatric Clinical Guidance on community-acquired neonatal sepsis and commenced on IV Amoxicillin, Cefotaxime & Gentamicin.
Rationalization
Once an organism has been isolated (assuming it is not considered a contaminant), rationalize the antimicrobial regimen to the narrowest spectrum that effectively covers the organism in the site from which it has been isolated. See below for specific organisms.
Duration - Consider stopping antibiotics:-
- Where symptoms have resolved - After 2 normal CRP samples at least 12 hours apart.
- Where symptoms persist – After 2 normal CRP samples at least 12 hours apart and blood culture is negative at 36 hours – Consider non-microbiological cause of the symptoms (30)(31)
- Following appropriate minimum duration of treatment as guided by organism (see table below), assuming satisfactory clinical response and normalization of CRP
Consider repeat cultures if there are ongoing signs/symptoms or rising CRP despite 24-48 hours of treatment. Central venous lines may need to be removed in Candidal infection or in Staphylococcal infections which do not respond to therapy
Consider stopping antibiotics after a minimum of:
- 5 days for Coagulase negative Staphylococci (CoNS) sepsis
- 7 days for non-CoNS septicaemia (but see also specific clinical situations below)
- 14 days, from date of first negative blood culture, for Staph. aureus septicaemia (see below)
- 14 days for Group B Streptococcal meningitis
- 21 days for Gram-negative meningitis
- 21 days for Listeria meningitis
- Mould and candida infections may require prolonged courses of antifungal therapy and individualised clinical plans should be prepared on the advice of microbiology.
see Table 1 for more detail
The decision to stop antibiotics should be guided by clinical response and serial CRP. If persisting symptoms/signs or abnormal CRP, consider:
- Repeating cultures (including urine, LP)
- Optimizing antibiotic doses and levels
- Further investigations (e.g. echo, X-ray, ultrasound) to look for a focus
- Changing to an alternative antibiotic e.g. Piperacillin/ Tazobactam (Tazocin) / Meropenem
- Adding Ambisome - blood cultures are often negative in fungal sepsis or only positive after 48 hours. Abdominal and cranial ultrasound, echo and ophthalmology review may reveal the presence of mycetomas.
- Complex cases may require consideration of diagnostic modalities such as fungal biomarkers and procalcitonin and should be discussed with microbiology
- Consider viral infections such as CMV in unresolving cases and discuss with virology for further guidance
Meningitis
Suspected: use Cefotaxime and Gentamicin in early onset sepsis, Cefotaxime and Vancomycin for late onset sepsis pending culture and microscopy results.
Gram positive: use Cefotaxime.
Cephalosporin resistance is rare and microbiological advice should be sought in such cases. Avoid vancomycin monotherapy as CSF penetration is variable (7–68%).(35)
Gram negative: use Cefotaxime and Gentamicin.
The adjunctive use of aminoglycosides for meningitis is controversial - CSF penetration in adults is poor, although it has not been studied in neonates. Nonetheless, clinical studies show efficacy with “once-daily” dosing.(35) 1 centre has reported excellent outcomes with combination treatment for gram negative meningitis in neonates (uncontrolled).(36)
N.B. If an Extended Spectrum Beta Lactamase organism, Enterobacter species, Pseudomonas or Serratia is identified, discuss alternative antibiotics with consultant microbiologist. Meropenem may be required for alternative CNS cover.
Intraventricular antibiotics are seldom required in the absence of underlying prosthetic hardware, devices, ventriculitis and neurosurgical issues, and can only be prescribed and administered by practitioners registered for the administration of intrathecal medications. Discuss with microbiology and local neurosurgical team on individual grounds.
Clearance samples of CSF may be considered to guide duration of therapy for infections with organisms such as Gram-negative bacilli, Listeria, and fungi and can be considered on a case by case basis.
Necrotising enterocolitis (NEC)
Babies with suspected or proven NEC should be on a standard combination of antibiotics appropriate for late onset sepsis. This should include an antibiotic effective against anaerobic organisms. Those infants who are already on Tazocin or Meropenem should have sufficient anaerobic cover, otherwise IV metronidazole should be added.
Listeria
Use Amoxicillin plus Gentamicin.
Although this is the most effective antibiotic regimen for listeriosis, the cure rate is only approximately 70%. These antibiotics only poorly penetrate the cerebrospinal fluid and thus high doses given over a prolonged period of 2-3 weeks are necessary. Discuss difficult or unresolving cases with microbiology.
Meconium staining of the amniotic fluid does not appear to be a useful indicator of Listeriosis where the incidence of infection is very low.(42) It may be more useful to assess avoidance of soft blue or mould ripened cheeses and paté during pregnancy.
Multidrug resistant Gram-negative organisms. These include:
- Extended-spectrum beta-lactamase (ESBL) producing organisms
- Gentamicin-resistant coliforms
- Carbapenemase producing organisms / Carbapenem resistant organisms (CPOs/CROs)
- Serratia and Enterobacter – these may not be reported as ESBLs, but cephalosporins are often inappropriate therapy. These organisms can also be associated with the environment as well as endogenous infection.
- Environmental organisms – these include Pseudomonas species, Chyseomonas, Stenotrophomonas, Acinetobacter, Elizabethkingia, Pantoea, Cupriavidus and others. Many of these organisms have intrinsically resistant antibiotic patterns, including resistance to gentamicin and meropenem.
Discuss alternative antibiotic regimen with consultant microbiologist
The emergence of extended spectrum beta lacatamase resistance has been associated with increased use of broad spectrum cephalosporins. Outbreaks of multi-resistant Klebsiella, Serratia and Enterobacter have been described in neonatal units.(38)(39) Mortality with such strains appears to be worse, not least due to the delay in initiating effective antibiotics pending laboratory identification.(8)(40)
Remember infection control measures. Pending surveillance cultures, consider temporarily amending the unit antibiotic policy regimen for late onset sepsis to include 1 antibiotic effective against the identified ESBLs or other multidrug resistant Gram-negatives if required based on local experience and epidemiology.(14) Such a move would require discussion with microbiology and IPC via appropriate governance mechanism.
Staphylococcus aureus
Methicillin-susceptible S. aureus (MSSA)
IV Flucloxacillin is the standard treatment. Duration for septicaemia is 2 weeks from the first negative culture.
- Maintain a high level of suspicion for this organism for
- Preterm infants on long term ventilation with a ventilatory deterioration and CXR signs of pneumonia or bullae
- Preterm infants unresponsive to first line treatment
- Line related infections unresponsive to first line treatment
- In relapsing/persisting infections, look for, osteomyelitis, abscesses or infected thrombus e.g. IV sites, deep veins, heart. Abscesses may require drainage.
- Deep vein thrombophlebitis, osteomyelitis or endocarditis require an extended treatment course e.g. 4-6 weeks.
- Flucloxacillin-sensitive Staphylococcus aureus strains may still harbor resistance to other antibiotics. Adjunctive treatment based on individual antibiotic resistance patterns and clinical circumstances may be required and should be discussed with microbiology.
Methicillin-resistant S. aureus (MRSA)
Vancomycin should cover MRSA although glycopeptide-resistant strains are emerging internationally. Targeted and adjunctive therapy should be discussed with microbiology.
Toxin-producing strains (such as PVL or TSST) - These are recognized to have enhanced virulence. If suspected, discuss with microbiology and infection control.
Anti- Fungal Prophylaxis
Dependent on local candida infection rates it may be appropriate to consider anti-fungal prophylaxis, such as Fluconazole, for high-risk infants. This may include babies who are very low birth weight and who have been on broad-spectrum or prolonged antibiotic regimens. Also where there is known colonization at multiple sites, or at a central line site.(32)(33). Please note that nonalbicans Candida species may harbor intrinsic resistance to fluconazole and should be discussed with the local microbiologist.
The decision to use anti-fungal prophylaxis will be made on an individual unit basis informed by local monitoring of infection rates. Fluconazole will not cover moulds, and the options of Amphotericin, echinocandins or other azoles should be discussed with microbiology if necessary. Empiric therapy for suspected fungal sepsis or where Candida isolated from culture is with Ambisome (liposomal Amphotericin is the commonest formulation but will not cover fungal infection of the renal/urinary tract). This can be narrowed to fluconazole or an alternative agent based on the individual organism and antifungal sensitivities.
Fluconazole is also effective against most Candida species and may be used as an alternative therapy following determination of sensitivity.
Prosthetic devices, hardware and centrally-placed lines
Bacteria form biofilm on prosthetic material. This can be difficult to treat with antibiotics alone, and removal of infected prosthetic material is generally recommended when possible.
In particular, intravascular catheter/long line removal is strongly recommended for S.aureus (MSSA or MRSA) infections, fungal infections and Gram-negative infections as these are highly virulent pathogens with a high chance of mortality or treatment failure in the context of retention of long lines.
N.B. For centrally-placed long lines that cannot be immediately removed, where removal will mean loss of IV access with a low chance of being able to re-site access urgently, or where line retention is deemed to be in the best interests of the patients, local policies for line salvage should be followed.
Please note that even if the primary focus of infection is elsewhere, long lines may become secondarily colonized and infected, and line locks should be considered as part of the management.
Patients with other complex hardware such as cardiac prostheses, extra-ventricular drains and ventriculoperitoneal shunts should be discussed early with microbiology and may require prolonged therapy with adjunctive antibiotics where hardware is retained.
Given that variations will be seen in factors such as protein binding and antibiotic sensitivity, before other adjuncts are considered always ensure that good doses of the appropriate antibiotics are being used, with good levels where measurable.
G/GM-CSF – no overall benefit is demonstrated for adjunctive or prophylactic use.(45)(46)(47)
Intravenous immunoglobulin - A large multi-center study of Intravenous immunoglobulin therapy for the treatment of neonatal sepsis (INIS) has found no benefit for the treatment of neonatal sepsis ((48) full publication awaited). This therapy is not indicated unless there is known to be a congenital deficiency of immunoglobulin production
Table 1 - Common Organisms and Recommended Antibiotics
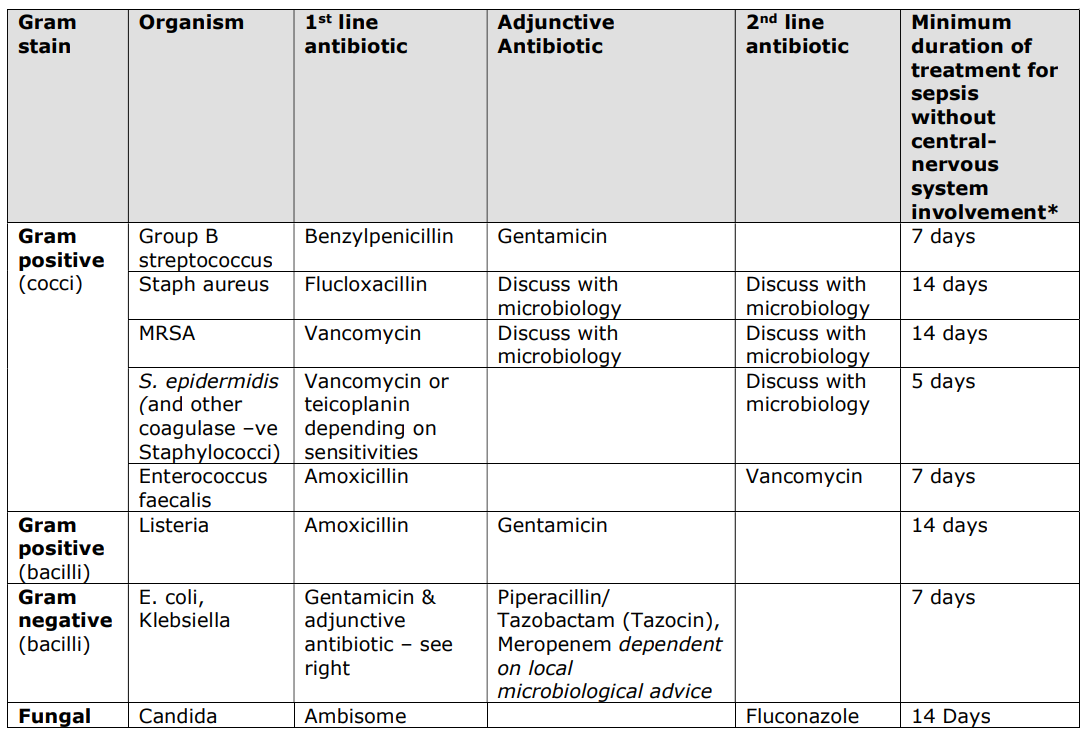
* as guided by clinical response, blood cultures, and serial CRP.
- Audit of local epidemiology
- Incidence of Early Onset Sepsis broken down by
- Site
- Organism
- Resistant strains
- Incidence of Late onset sepsis
- Organism
- Resistant strains
- Line related sepsis
- Incidence of Early Onset Sepsis broken down by
- Duration of antibiotic therapy in infants without CRP rise and without positive culture.



