Anti-Thrombotic therapy, Haematology
Objectives
Anti-thrombotic protocol.
The following sections give the current departmental guidelines for anticoagulation, reversal of warfarin and thrombolysis.
Scope
These protocols should be used by healthcare professionals involved in the management, prescribing and administration of anticoagulant medications in children.
During working hours, problems regarding thrombosis should be discussed with the consultant on for non-malignant haematology - Dr Chalmers (Deg 85644), Dr Pinto (Deg 85646) or the Haematology SpR (page 85645).
Out of hours calls should be discussed with the Haematology Consultant on call for the Laboratory Haematology (contact via switchboard).
The following sections give the current departmental guidelines for anticoagulation, reversal of warfarin and thrombolysis.
3.1 The management of thrombotic problems will be directed by the Consultant or a senior member of the medical team.
3.2 The Medical/Nursing team will be responsible for admitting, assessing, investigating and administrating treatment, and monitoring response.
None
Refer to the Vascular Access Procedure and Practice guideline.
5.2.1 Loading dose: UFH 75 units/kg IV over 10 minutes.
5.2.2 Initial maintenance dose:
- 25-28 units/kg/hour for infants < 1 year
- 20 units/kg/hour for children > 1 year
5.2.3 Adjust UFH to maintain APTT 60-90 seconds (assuming this reflects an anti-factor Xa level of 0.35 to 0.70)
5.2.4 Obtain blood for APTT 4-6 hours after administration of the heparin loading dose and 4-6 hours after every change in the infusion rate (for guidance on dose adjustments see Table 1).
5.2.5 When APTT values are therapeutic, monitor with daily FBC and twice daily APTT.
5.2.6 If the baseline APTT is prolonged prior to commencing heparin therapy or there are problems achieving a satisfactory APTT consider monitoring using Anti-Xa levels (discuss with haematology).
5.2.7 Failure to achieve a therapeutic APTT despite high doses of heparin may be an indication to check antithrombin activity (discuss with haematology).
5.2.8 Check the platelet count prior to heparin and daily thereafter during heparin therapy. If the platelet count drops by >30% from baseline consider heparin induced thrombocytopenia (HIT), this is more likely after 5-10 days of treatment. Investigate other causes of thrombocytopenia and if HIT remains a possibility consider HIT antibody screen (discuss with haematology). For management of HIT see Adult HIT guideline – “Diagnosis and Treatment of Heparin Induced Thrombocytopenia”
| Stage | Description | APTT | Bolus U/kg | Withhold, min | Rate change | Repeat APTT |
| I | Loading dose | 75 IV over 10 min | ||||
| II | Initial maintenance dose Infants < 1 yr Children > 1 yr |
28/h 20/h |
||||
| III | Adjustment | <50 50-59 60-90 90-99 100-120 >120 |
50 0 0 0 0 0 |
0 0 0 0 30 60 |
+10% +10% 0 -10% -10% -15% |
4hr 4hr Next day 4hr 4hr 4hr |
| IV | Obtain blood for aPTT check 4hr after heparin loading dose and 4hr after every change in infusion rate. |
|||||
| V | When aPTT values are in therapeutic range, perform daily FBC and APTT measurement |
If anticoagulation with UFH needs to be discontinued for clinical reasons, termination of the heparin infusion will usually suffice due to the rapid clearance of heparin. If an immediate effect is required IV protamine sulphate will reverse heparin therapy.
The dose of protamine required is based on the amount of heparin received in the preceding 2 hours (see below & also BNF).
Time since last heparin dose: <30 minutes
Protamine dose: 1.0 mg/100 units heparin received
Time since last heparin dose: 30- 60 minutes
Protamine dose: 0.5-0.75 mg/100 units heparin received
Time since last heparin dose: 60-120 minutes
Protamine dose: 0.375-0.5 mg/100 units heparin received
Time since last heparin dose: >120 minutes
Protamine dose: 0.25-0.375 mg/100 units heparin received
Maximum dose of 50 mg
Infusion rate of a 10 mg/ml solution should not exceed 5 mg/min.
Hypersensitivity reactions to protamine sulphate may occur in patients with known hypersensitivity reactions to fish or those previously exposed to protamine therapy or protamine-containing insulin.
AGE-DEPENDENT DOSE OF ENOXAPARIN (CLEXANE):
Initial treatment dose
Neonate: 1.5-2mg/kg dose bd
Age 1-2 months: 1.5 mg/kg/dose bd
Age > 2 months: 1.0 mg/kg/dose bd
Initial prophylactic dose
Age < 2 months: 0.75 mg//kg/dose bd
Age > 2 months: 0.5 mg/kg/dose bd (max dose of 40mg per day)
Monitoring:
- Therapeutic levels of LWMH are based on an anti-Xa level of 0.5-1.0 U/ml at 4 hours post dose. Prophylactic levels are 0.2-0.4 U/ml.
- Order ‘control of heparin – child’ on trakcare
- The platelet count should be monitored during LMWH therapy.
- Dose adjustments may be required following initiation of therapy
- In children with stable renal function, once levels are within the therapeutic range the frequency of monitoring can be reduced to every 1-2 weeks
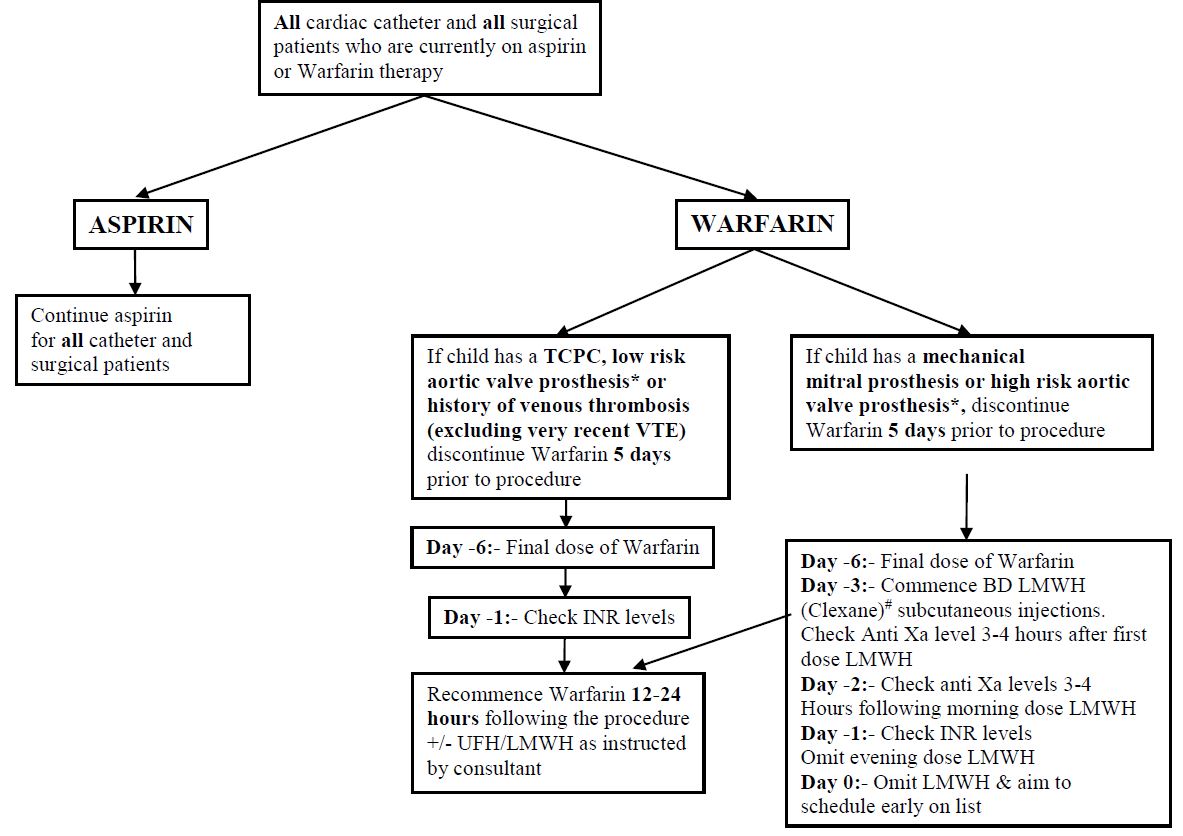
I. Loading day 1: If the baseline INR is 1.0 to 1.3:
Dose = 0.2 mg/kg orally (max dose 10mg)
II. Loading days 2-4: If the INR is:
| INR | ACTION |
| 1.1-1.3 | repeat initial loading dose |
| 1.4-1.9 | 50% of initial loading dose |
| 2.0-3.0 | 50% of initial loading dose |
| 3.1-3.5 | 25% of initial loading dose |
| >3.5 | hold until INR <3.5 then restart at 50% less than theprevious dose |
Induction dose may need to be altered according to condition (e.g. abnormal liver function tests, cardiac failure), concomitant interacting drugs, and if baseline INR above 1.3
III. Maintenance Oral Anticoagulation Dose Guidelines for INR 2-3:
| INR | ACTION |
| 1.1-1.4 | increase by 20% of dose |
| 1.5-1.9 | increase by 10% of dose |
| 2.0-3.0 | no change |
| 3.1-3.5 | decrease by 10% of dose |
| >3.5 | hold until INR <3.5 then restart at 20% less than the previous dose |
The management of low INRs is not evidence based, the following advice is therefore pragmatic and is similar to practice at other UK paediatric centres.
For patients in the HR group it will be useful for parents to have been instructed on the administration of LMWH.
LMWH dose: enoxaparin (Clexane) 1mg/kg bd.
Children with Cardiac indications.
Low risk e.g. Fontan/TCPC with no PHx of thrombosis.
- INR range 2-3: INR < 1.5 consider LWMH for persisting low INR values
High risk e.g. mechanical valves, children with previous thrombosis.
- INR range 2-3: INR < 1.8 start LMWH
- INR range 2.5-3.5: INR < 2.0 start LMWH
- INR range 3-4: INR < 2.5 start LMWH
Children with VTE.
- INR range 2-3: INR < 1.5 consider LMWH if VTE is within the previous month.
General points regarding monitoring:
- Coagucheck monitors do not accurately define high INR values. If INR >6 a repeat venous sample will help to define the degree of over anticoagulation.
- Coagucheck monitors should not be used to monitor children receiving concomitant heparin/LMWH therapy.
- Coagucheck monitors should not be used in children with a HCT > 0.55.
|
INR |
Action |
|
3.0 < INR < 6.0 (target INR = 2.5) |
Reduce Warfarin dose or stop |
|
6.0 < INR < 8.0 |
Stop Warfarin |
|
INR > 8.0
|
Stop Warfarin |
|
Major bleeding (any INR) or requiring emergency surgery
|
Stop Warfarin
|
Table 1:
|
INR |
Approximate Dose |
|
2.0-3.9 |
1 ml/Kg = 25 iu/Kg |
|
4.0-6.0 |
1.4 ml/Kg = 35 iu/Kg |
|
>6.0 |
2 ml/Kg = 50 iu/Kg |
Ref: BCSH Guidelines on oral anticoagulant therapy, Br J Haematol 2011
- For patients on short term anticoagulant therapy consider delaying dental surgery until treatment has been completed.
- Prior to dental procedure stop or adjust anticoagulant therapy to achieve an INR of approximately 2.0 on the day of surgery.
- The INR should be checked 5-7 days prior to the planned dental procedure and dose adjustments made as required by haematology/cardiology staff.
- The INR should be checked again pre-operatively on the day of the procedure.
- If the INR is < 2.5 proceed with dental work.
- If the INR is > 2.5 discuss with haematology/cardiology SpR/Consultant.
- For older children the use of tranexamic acid mouthwash (4.8% may be helpful).
Re-establish usual anticoagulant dose post operatively and arrange follow up anticoagulant
Ref:BCSH Guideline on oral anticoagulant therapy, Br J Haem 1998;101:374-387, Dental Practitioners formulary 2002-2004. D8.
ADDITIONAL NOTES REGARDING PRE-OP MANAGEMENT
*AORTIC VALVE PROSTHESES:
Low risk: A standard bi-leaflet aortic valve prosthesis with no additional risk factors is considered low risk.
High risk: An increased risk of thrombosis may result from a number of factors including the following:
- Previous thromboembolism
- Left ventricular dysfunction
- Hypercoagulable conditions
- Atrial fibrillation
Therapeutic LMWH should be administered for bridging in these situations as per the pathway for a mitral valve prosthesis.
#THERAPEUTIC DOSE OF LMWH HEPARIN (CLEXANE) FOR BRIDGING UNTIL CATHETER/SURGERY:
>2 months 1mg/kg BD
<2 months 1.5-2mg/ kg BD
INR LEVELS
If the INR is >1.5 when checked on day -1, inform the patient’s consultant, so a decision for reversal can be made.
If reversal is appropriate give:
- Vitamin k 30mcg/kg IV or oral
- If given orally, use Konakion MM preparation
Supporting documentation for this pathway is available on request.
See Appendix 1 for guidance and tables.
TISSUE PLASMINOGEN ACTIVATOR (t-PA):
Dose: 0.1-0.6 mg/kg/hr as an IV infusion for 6 hours.
Lower doses may also be used in specific situations – discuss with on call haematologist.
Monitoring: Monitor FBC and coagulation screen (including D-Dimers) every 3 hours. Maintain platelets > 100 and fibrinogen > 1g/dl
Start heparin (UFH) or LWMH therapy upon completion of thrombolytic therapy. A loading dose of heparin may be omitted.
Thromboprophylaxis should be considered in all adolescents on admission, and also during their period of stay in hospital.
A suitable tool for risk assessment in adolescents is available – see Appendix 2.
Advice of the need for thromboprophylaxis may be discussed with the clinical team directly managing the patient and with haematology.
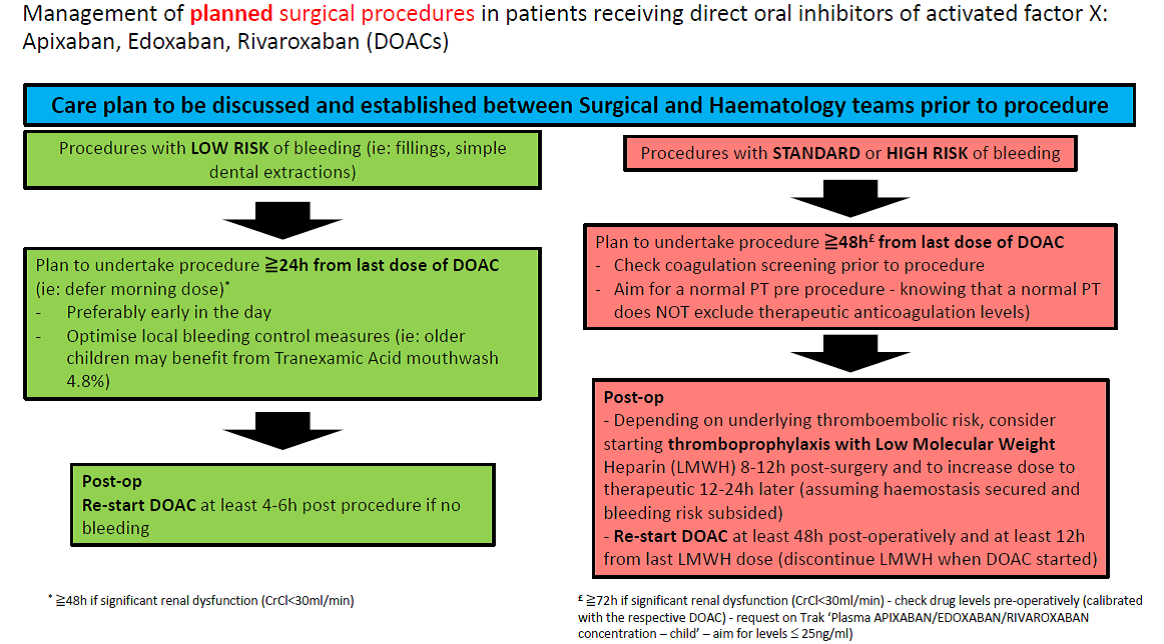
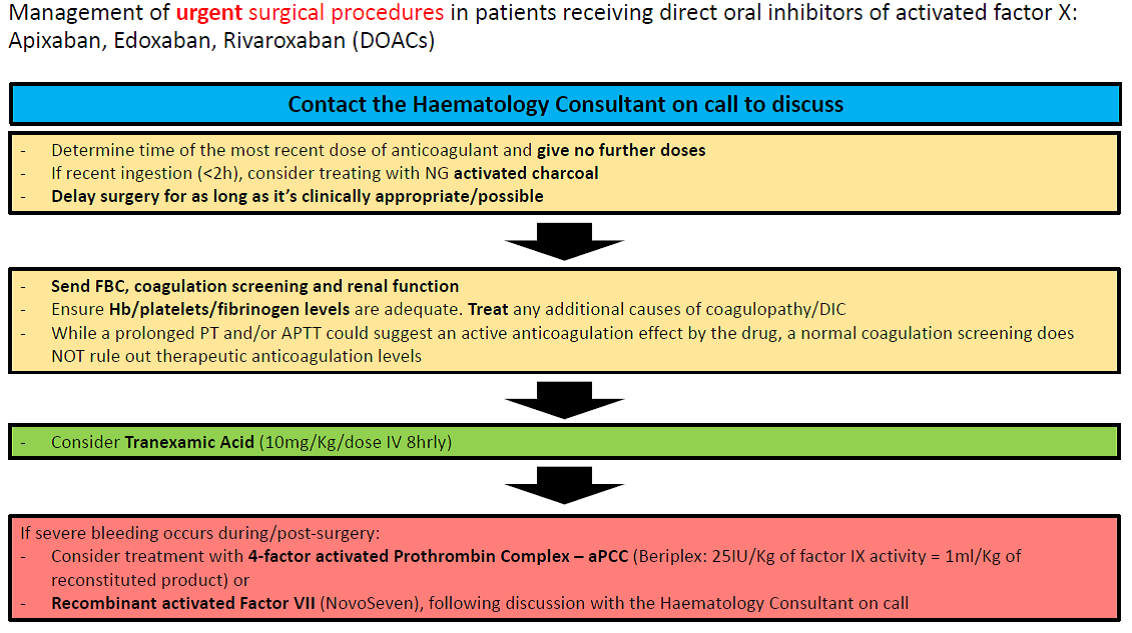
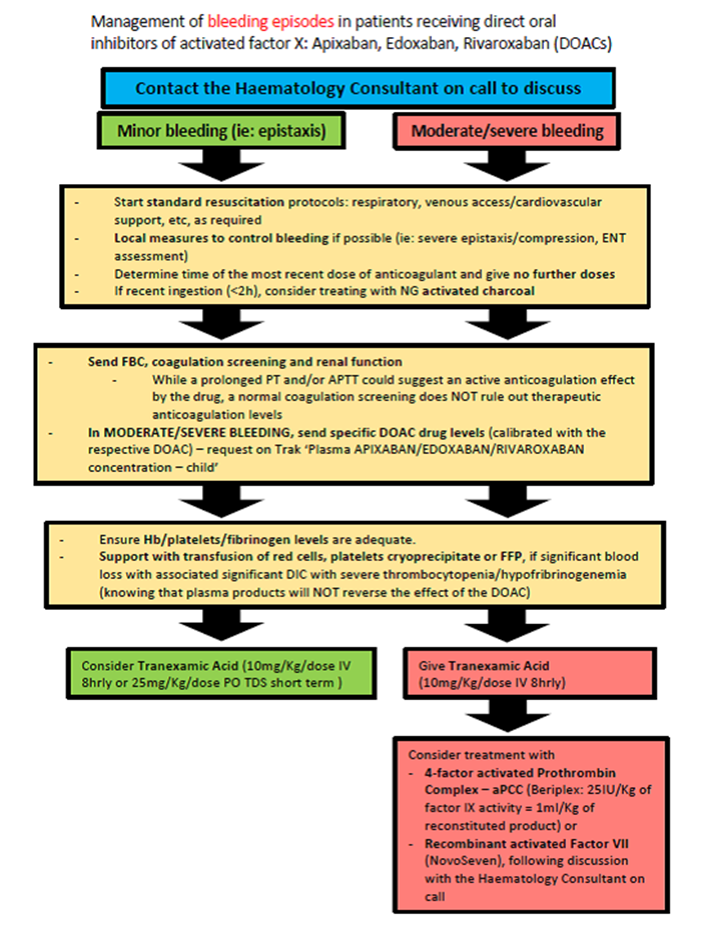
Management of bleeding and surgery in patients receiving Direct Oral Anticoagulants (DOACs): Apixaban, Edoxaban, Rivaroxaban
Apixaban, Edoxaban and Rivaroxaban are oral direct inhibitors of activated factor X which are now being investigated in children in Clinical Trial protocols. None as yet licenced for patients < 18yrs of age.
Unlike Warfarin and Heparin, they do not normally require monitoring of levels and have a relatively short half-life of 8-12h (although this can be longer in significant renal dysfunction and they are not dialysable).
While a prolonged PT and/or APTT could suggest an active anticoagulation effect by the drug, a normal coagulation screening does NOT rule out therapeutic anticoagulation levels. Therefore, knowing the time since last dose, whether there is potential prolongation of the expected standard half-life (ie: due to drug interactions or organ dysfunction) and sometimes determining the specific Anti-Xa/drug levels (calibrated with the respective DOAC), may all be needed to help with estimating the DOAC effect at any given moment.
No specific antidotes for these agents currently exist in clinical practice so management of bleeding generally involves discontinuation of the drug and general haemostatic supportive measures.
Management of planned surgical procedures in patients receiving direct oral inhibitors of activated factor X: Apixaban, Edoxaban, Rivaroxaban (DOACs):
Management of urgent surgical procedures in patients receiving direct oral inhibitors of activated factor X: Apixaban, Edoxaban, Rivaroxaban (DOACs):
Management of bleeding episodes in patients receiving direct oral inhibitors of activated factor X: Apixaban, Edoxaban, Rivaroxaban (DOACs):
Last reviewed: 31 January 2021
Next review: 31 January 2023
Author(s): Dr E Chalmers
Version: 5
Approved By: Schiehallion Clinical Governance Group
Document Id: RHC-HAEM-ONC-007