Fluid and electrolytes (Haemato-oncology patients, Schiehallion)
exp date isn't null, but text field is
The following guideline is intended as a basic introduction to fluid, nutrition and electrolyte management within the haematology/oncology department. Each section should be read in conjunction with any relevant RHC clinical guidelines.
3.1 The management of patients will be directed by the Consultant/Associate Specialist or a senior member of the medical team.
3.2 The Medical/Nursing team will be responsible for admitting, assessing, investigating and administrating treatment, and monitoring response (see Appendix 1).
None.
MINIMUM DAILY REQUIREMENTS
| FLUIDS | CALORIES | |
| Up to 10kg | 100ml/kg |
100kcal/kg |
| Over 10kg | 1000ml + 50ml/kg over 10kg |
1000kcal + 50kcal/kg over 10kg |
| Over 20kg | 1500ml + 20ml/kg over 20kg |
1500kcal + 20kcal/kg over 20kg |
- Total volume of fluids per day should not exceed 4500ml in any patient
- Use an accurate and up-to-date body weight to calculate fluid requirements
- If the patient is very overweight, fluid is prescribed as per weight, no capping done, except the daily limit of 4500ml
- If hyperhydration is required this should be calculated by body surface area (BSA) and not weight. In this case BSA should be capped for extremely overweight patients and adjusted to second centile for underweight patients. BSA charts are available on the ward.
Type of Intravenous Fluids:
There are 4 main types of intravenous fluid used within the haematology/oncology unit (see Appendix 2).
- Sodium Chloride 0.45% and Glucose 2.5%
This is the standard hydration fluid within the unit – the sodium content is approximately 77mmol/L. Note that Sodium Chloride 0.45% and Glucose 5% is widely used throughout RHC and may be safely administered to many children, however as haematology/oncology patients often require hyperhydration, the large fluid volumes may result in an excessive carbohydrate load leading to issues with blood glucose control.
- Plasma-Lyte 148
Plasma-Lyte is an iso-osmolar (pH 7.4) solution for intravenous infusion which contains sodium 140mmol/L, Potassium 5mmol/L & Magnesium 1.5mmol/L – its constituents are designed to match those of plasma. It also contains acetate & gluconate so is therefore a mild alkalinizing agent. Plasma-Lyte is used widely throughout RHC and may be appropriate in some haematology/oncology patients. However, there is currently no stability data available to run Plasma-Lyte concurrently with chemotherapy, therefore it should not be used as hydration in any child receiving chemotherapy. In addition, SPC advises caution in use in renal failure or in hyperkalaemia, therefore it should not be used as hyperhydration in a tumour lysis prevention protocol.
- Glucose 5%
This is used as the base fluid in children receiving high dose Methotrexate and requiring alkalinisation because of the excess sodium added to the bag as Sodium Bicarbonate. The addition of Sodium Bicarbonate 8.4% 50mmol/L results in a solution approximately equivalent to Sodium Chloride 0.45% and Glucose 5%
- Sodium Chloride 0.9% (Isotonic saline)
This is an isotonic fluid which contains 9 g/L Sodium Chloride and has an osmolarity of 308 mOsmol/L (approx). It contains 154 mmol/L sodium and 154 mmol/L chloride. This is the solution of choice for those with potential risk of or established hyponatraemia.
In some circumstances, children should be administered other isotonic fluids such as glucose 5%, or Hartmann’s solution/Ringer-Lactate solution. Therefore IV fluid choice should be tailored to each patient’s individual needs. These circumstances include:
- peri and immediate post operative patients
- if plasma sodium is at the lower normal reference range
- intravascular volume depletion
- hypotension
- sepsis
- excessive gastric, stoma or diarrhoea loss
- salt-wasting syndromes and chronic conditions such as diabetes, cystic fibrosis, adrenal insufficiency, pituitary deficits and those requiring replacement of ongoing losses
Check blood electrolytes and glucose when starting IV fluids and at least every 24 hours thereafter
The nutrition team in the hospital will accept referrals for advice and management of difficult patients. Pharmacy will advise on initiating and incrementing PN, and each patient should be discussed daily with a ward pharmacist.
Basic Components:
These are general guidelines. individual patients may require more or less than the suggested target values given.
Most paediatric parenteral nutrition is presented as two separate solutions – an aqueous phase containing amino acids, carbohydrate and electrolytes, and a separate lipid phase. This is generally a result of stability issues in mixing aqueous & lipid phases within the volumes used in paediatric patients
| Amino Acids |
Increase slowly over 2 – 3 days to:
An age appropriate protein source will be selected by preparative services, there is no need to specify. |
| Carbohydrate |
Start at Glucose 10% and increase as appropriate
|
| Fat |
Start at 0.5 – 1g/kg/day and increase as tolerated –
|
Electrolytes – Daily Requirements:
|
|
Up to 10kg |
10 – 20kg |
20kg+ |
|
Na (mmol/kg) |
2.5 |
2.5 |
2.5 |
|
K (mmol/kg) |
2.5 |
2.5 |
2.5 |
|
Ca (mmol/kg) |
0.5 |
0.25 |
0.2 |
|
Mg (mmol/kg) |
0.25 |
0.2 |
0.2 |
|
PO4 (mmol/kg) |
0.5 |
0.5 |
0.25 |
These are considered as baseline electrolyte requirements and can be used as a guide when initiating PN. Consider what electrolytes the patient has received from IV fluids in the 24 hours prior to starting parenteral nutrition as baseline figures given above may not be sufficient. Also consider factors that might promote loss and lead to increased requirements (e.g. AmBisome, diuretics etc.)
Trace elements:
- <15kg Peditrace 1ml/kg or 1ml/100ml (whichever is less - max 10ml)
- >15kg Additrace 0.25ml/kg/day (max 10ml)
- Trace elements are added automatically
- If, for any reason, you specifically do not want trace elements added, make this clear
- Caution in renal/hepatic impairment – consider giving trace elements twice-weekly only
- Trace element screen should be done after approx. 1 month on PN, then repeated at monthly intervals.
**NB: A high CRP affects assay results for trace elements – try to assay at a time when CRP is not elevated (if possible!)**
Vitamins:
- Solivito (water-soluble Vitamins B1,2,6 & 12, Vitamin C, Folic Acid)
Added automatically at day 1 of PN. Solivito is added at 1ml/kg or 1ml/100ml (whichever is less – max 10ml)
- Vitlipid (fat-soluble vitamins A,D,E & K)
Added automatically once lipid started. Vitlipid Infant 4ml/kg (max 10ml) is used until 11 years old then Vitlipid Adult 10ml added
In patients who are not able to tolerate lipid for an extended period discuss with pharmacy. Cernevit may be used which contains both water and fat soluble vitamins, but note this preparation does not contain Vitamin K.
Ordering:
- Order PN Mon – Thurs on a daily basis
- Order three days PN Fri for weekend/public holidays (festive holidays may differ – discuss with pharmacy)
- Pharmacy will liaise with ward medical staff every day (Mon – Fri) for prescriptions
- PN MUST BE CONFIRMED BY 1pm AT THE LATEST
- All PN patients should have daily U/Es, LFTs, Mg, Bone Profile, Glucose & Triglycerides ordered
- It is the responsibility of ward medical staff to ensure that biochemistry results are available in order to safely make decisions on an appropriate PN prescription
- Aqueous PN and lipid should be prescribed daily on individual patient fluid prescription sheets.
Sodium:
Hyponatraemia is common in paediatric haematology/oncology patients, often as a result of inappropriate administration of free water intravenously. A syndrome of inappropriate antidiuretic hormone (SIADH) secretion is a common cause of hyponatraemia, particularly in brain tumour patients. It is also frequently associated with Vincristine therapy (exacerbated by concomitant administration of azole antifungal drugs). See Appendix 2 for table listing common drugs used in haemato-oncology patients that can cause SIADH (note that arginine vasopressin is also called antidiuretic hormone).
- SIADH can also be seen in patients with respiratory conditions and/or other infections.
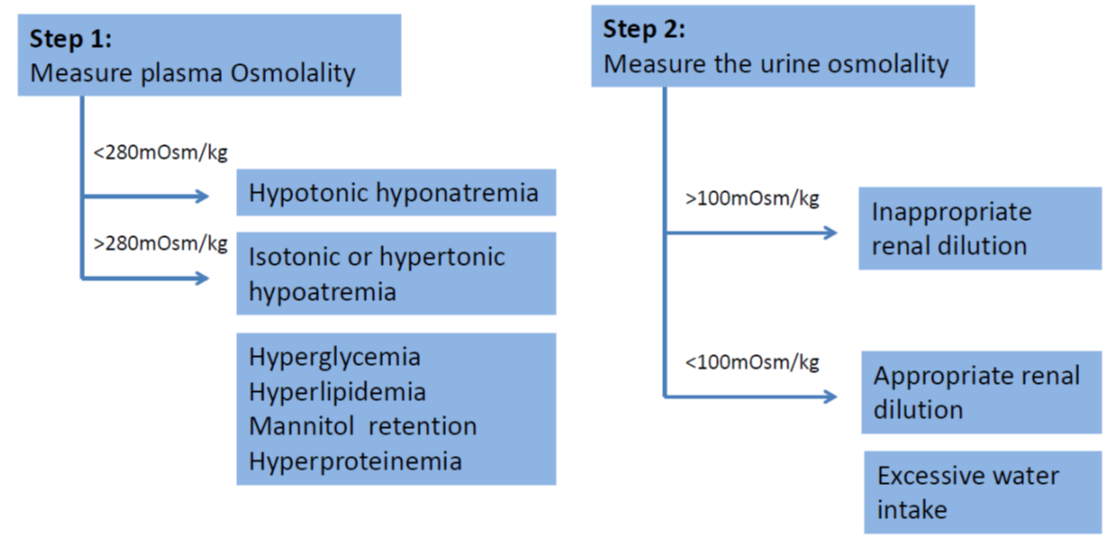
- Beware of pseudohyponatremia which is a condition where serum sodium is <135mmol/L, but serum osmolality is maintained within normal limits (280-295 mOsm/kg H2O). Pseudohyponatraemia is caused by a displacement of serum water by elevated serum lipid (hyperlipidemia) or proteins (hyperproteinemia), or in cases of hyperglycaemia which causes increased movement of water out of cells. These patients should be considered as in isonatremic.
- SIADH is diagnosed when the following criteria are met (taken from Bartter & Schwartz in 1967):
|
Criteria for Diagnosing SIADH |
|
Decreased effective osmolality of the extracellular fluid (Posm <275 mOsmol/kg H2O). Inappropriate urinary concentration (Uosm >100 mOsmol/kg H2O with normal renal function) at some level of plasma hypo-osmolality. Clinical euvolemia, as defined by the absence of signs of hypovolemia (ortostasis, tachycardia, decreased skin turgor, dry mucous membranes) or hypervolemia (subcutaneous odema, ascites). Elevated urinary sodium excretion (>20-30 mmol/L) while on normal salt and water intake. Absence of other potential causes of euvolemic hypo-osmolatlity: severe hypothyroidism, hypocortisolism (glucocorticoid insufficiency). Normal renal function and absence of diuretic use, particularly thiazide diuretics. |
|
H2O = water; kg = kilogram; mmol = millimole; mOsmol = milliosmole; Posm = plasma osmolality; SIADH = syndrome of inappropriate antidiuretic hormone secretion; Uosm = urine osmolality |
- Decreased effective osmality of the extracellular fluid
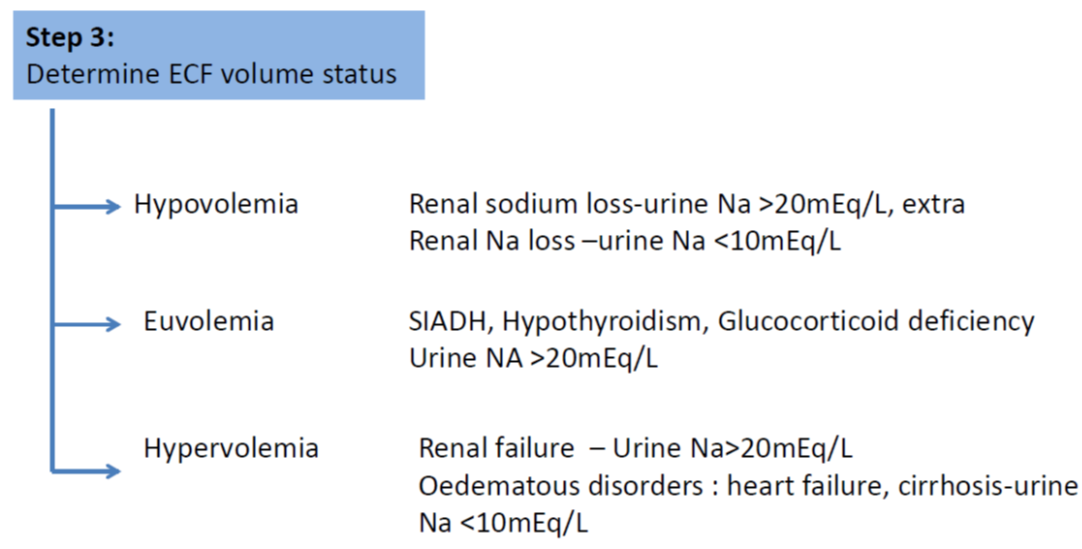
- Ongoing sodium losses from vomitus, diarrhoea or stomas are frequently seen but renal leak is rare
- Plasma and urinary electrolytes and osmolality MUST be obtained and a cause of hyponatraemia must be established before effective therapy can commence
- A clinical evaluation of the patient’s hydration status is mandatory and this should include regular weights as well as examination of the fluid balance
- Correcting the cause of hyponatraemia (frequently by fluid restriction rather than sodium supplementation) is the cornerstone of the management of hyponatraemia
- Most hyponatraemic patients can be managed safely and appropriately with fluid restriction
- Patients may become symptomatic when plasma sodium is <130mmol/L. Serious complications are more likely at plasma sodium levels <125mmol/L or following a rapid fall in plasma sodium.
- If Sodium supplementation is necessary:
Dose
Acute supplementation:
- Na required = (desired Na – observed Na) x 0.6 x weight(kg) (mmol)
- Replace SLOWLY over 24 – 48 hours
- Maintenance fluids (Sodium Chloride 0.9%) are sufficient for most deficits
- Maintenance: 5 – 5mmol/kg/day
Preparations
| IV |
Sodium Chloride 0.9% = Na 154mmol in 1000ml |
| ORAL |
Sodium Chloride SR 600mg = Na 10mmol + Cl 10mmol (Slow Sodiumâ) Oral supplementation is unpleasant and rarely necessary. Patients should not be discharged on Sodium supplements without discussion with the consultant concerned and plans for routine Biochemistry monitoring. |
| A detailed management of symptomatic hyponatraemia and trust policy on management can be found here. | |
Potassium:
Supplement if K < 3mmol/L OR if symptomatic
Consider sources of ongoing losses, drug therapy etc before decision to treat.
|
Dose Acute supplementation:
Maintenance: |
0.2mmol/kg/hr 1 - 2mmol/kg/day |
|
Administration Standard volume: Minimum volume: |
usual max. conc. 40mmol/L **CENTRAL ADMINISTRATION ONLY** |
In exceptional circumstances a maximum concentration of 200mmol/L (ie 20mmol in 100ml) could be used following discussion with patients’ consultant and pharmacy. This equates to the maximum stable concentration which could be administered in a parental nutrition solution for central administration.
**Also consider use of K-sparing diuretics e.g. amiloride **
Preparations
| IV |
Potassium Chloride 15% = K 2mmol/ml |
|
NB Potassium chloride 15% solution MUST ONLY be added to plain infusion bags. NEVER add additional potassium to a ready to use preparation containing potassium. |
|
| ORAL |
Tablets Liquid |
| Patients should NEVER be discharged home on amiloride AND potassium supplements without consultant authorisation. | |
Calcium:
Supplement if corrected Ca < 2mmol/L OR if symptomatic.
Rarely necessary – consider sources of ongoing losses before decision to treat
|
Dose Acute supplementation: Maintenance: |
0.07mmol/kg 0.25 – 1mmol/kg/day (in divided doses) |
|
Administration Standard volume: Minimum volume: Standard rate: Maximum rate: |
Dilute 1 in 5 (0.045mmol/ml) Give undiluted (NB Central Line only) 0.0225mmol/minute Urgent supplementation: slow bolus over 5–10 mins |
Preparations
| IV |
Calcium Gluconate 10% = Ca 0.225mmol/ml |
| ORAL |
Tablets Dispersible Tablets Liquid |
Magnesium:
Supplement if Magnesium <0.6mmol/kg OR if symptomatic
Consider sources of ongoing losses, drug therapy etc before decision to treat
|
Dose Acute supplementation: Maintenance: |
0.2mmol/kg 0.2-0.25mmol/kg/day |
|
Administration Standard volume: Standard rate: Maximum rate: |
Dilute 1 in 5 (0.4mmol/ml) Slow IV bolus over at least 10 mins Slow bolus for smaller doses (up to 8mmol) |
Preparations
| IV: |
Magnesium Sulphate 50% = 2mmol/ml Mg |
|
ORAL:
|
Tablets Liquid |
Phosphate:
Supplement if phosphate < 0.7mmol/kg OR if symptomatic
This is rarely necessary, hypophosphataemia is usually dilutional. Tubular phosphate leak must be proven prior to consideration of supplementation. There is very poor compliance with oral phosphate supplements because of their unpleasant taste & side effects.
|
Dose Acute supplementation: Maintenance: |
0.3-1mmol/kg/day 0.15 – 0.3mmol/kg/day |
|
Administration Standard volume: Standard rate: Maximum rate: |
Dilute to 0.1mmol PO4/ml 0.05mmol/kg/hour IN EMERGENCY ONLY |
Preparation
| IV: |
Potassium Acid Phosphate 13.6% = 1mmol/ml NB INTRAVENOUS PREPARATIONS CONTAIN POTASSIUM - SAFETY GUIDELINES AS FOR POTASSIUM CHLORIDE APPLY |
| ORAL: |
Tablets Liquid |
|
Drugs known to cause hyponatremia by affecting arginine vasopressin (AVP) production or action |
|
|
Mechanism |
Drugs |
|
|
|
|
|
* Cisplatin may also cause hyponatremia by damaging renal tubules and interfering with sodium reabsorption. Adapted from Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis 2008;52:144-153 |
|
BNF/ BNF-C (access via www.medicinescomplete.com)
www.clinicalguidelines.scot.nhs.uk/ggc-guideline-library
National Institute for Health and Care Excellence (NICE) guidelines (www.nice.org.uk)
Evelina London Paediatric Formulary
Handbook on Injectable Drugs (Trissel LA) American Society of Health-System Pharmacists (access via www.medicinescomplete.com)
Minimum Infusion Volumes for fluid restricted critically ill patients (4th Edition Dec 2012). www.ukcpa.org
Last reviewed: 01 March 2020
Next review: 31 March 2023
Author(s): Carol Mitchell & J Sastry
Version: 3
Approved By: Schiehallion Clinical Governance Group
Document Id: RHC-HAEM-ONC-008