Venesection for iron overload
exp date isn't null, but text field is
Objectives
Iron overload can lead to multiple organ dysfunction/failure and poor quality of life in a variety of patients with different haematological conditions, including haemoglobinopathies, polycythaemias, haemochromatosis and post haematopoietic stem cell transplant. Patients entering into haematopoietic stem cell transplantation with iron overload have an increased risk of delayed engraftment, veno-oclusive disease (VOD), infection and GVHD.
In addition to oral or parenteral iron chelation, venesections may also be used as a treatment modality for the management of iron overload in selected patients. This SOP aims to define indications and contra-indications for the use of venesections in this clinical scenario, the competencies and equipment required, and to describe the procedures to be followed.
Scope
Children at risk of iron overload.
Audience
AUTHORISED PERSONNEL/SPECIFIC STAFF COMPETENCIES
- Daycare medical staff and senior nurse
- Cannulation skills
- Clinical assessment skills
Whatever the aetiology (ie: multiple transfusions, increased iron absorption in haemoglobinopathies or haemochromatosis), the diagnosis of iron overload and estimation of its clinical impact (ie: iron content in tissues, organ dysfunction) should include at least the following:
- FBC, blood film
- Ferritin, serum iron, transferrin, transferrin saturation, CRP
- Renal and liver functions, Hepatitis B/C serologies
- Endocrine assessment (i.e.: thyroid function tests) if any symptoms suggesting dysfunction of specific systems are present
- HFE genotype status
- If the child/young person can tolerate, also an MRI of the heart/liver without sedation for estimation of iron content
Consider intervention/treatment for the following patients:
- Serum ferritin > 1000 microg/L
- Transferrin Saturation > 50%
- Estimated liver iron > 5 mg/d dry weight by MRI
- Cardiac T2* MRI < 20 msec
- Any sign of iron-related dysfunction of target organs (liver, heart, hypophysis, thyroid, pancreas). In these cases, biopsy of the relevant organ to correlate dysfunction with iron overload may be necessary
Patients can be offered venesection if:
- They have documented iron overload needing treatment, as above
- Hb is within normal limits (although if Hb levels are borderline and venesections needed and still thought to be the most clinically appropriate treatment modality for controlling the iron overload, consideration should be given to the concurrent use of erythropoietin)
- There is no clinical contraindication from the cardiovascular point of view for the procedure to be done
- (Verbal) patient/parent consent to procedure available
The venesection will not proceed if the patient has an intercurrent viral infection. Delay venesection until fit to proceed. If the child is unwell, treat as appropriate for illness.
- Scales
- Recliner chair or bed/trolley
- Equipment for cannulation (Blue cannula-23 gauge)
- Extra syringes
- Blood bottles and forms for biochemistry and haematology
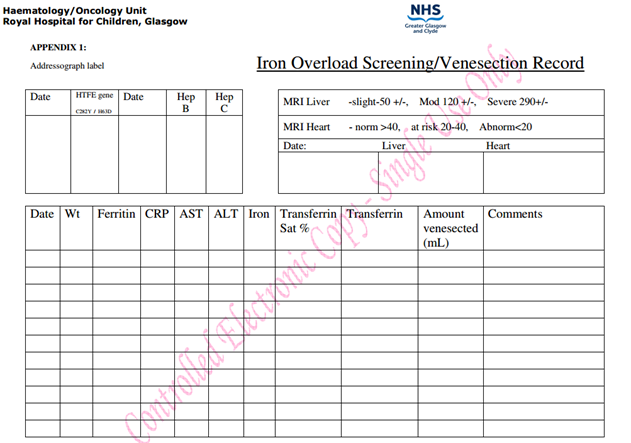
- Venesection chart in case-records (see Appendix 1)
This procedure involves the removal of a specified amount of blood by venepuncture, every 3 to 4 weeks and as tolerated, to control iron overload. Venesections should continue, if well tolerated, until ferritin reaches values < 300 microg/L or the liver iron concentration is <5mg/g dry weight.
Prior to each venesection procedure the following should be done, as a baseline:
- Weight, baseline temperature, pulse, respirations and blood pressure;
- Ensure patient is well clinically and that there are no active clinical issues of concern;
- Send FBC, reticulocytes, U&Es, LFT’s, serum iron, transferrin, transferrin saturation, ferritin, CRP and document these in the iron overload screening chart (see Appendix 1)
Ensure that the patient is fully informed of the procedure and any questions are answered.
If there is a history of fainting - lie flat for procedure and 30 minutes after completion.
The nurse must always ensure that the patient is feeling well and that he/she has eaten as hunger/dehydration can lead to fainting during or post procedure.
The patient’s blood count must be checked prior to carrying out procedure and results from last venesection (e.g. ferritin and iron levels).
The amount of blood to be venesected must be calculated prior to each venesection based on the patient's weight (5-7mls/kg to max 350mls) or previous amount venesected/comments on chart (e.g. fainted after 200mls taken)
Patient is cannulated and venesected over 30 mins or slower.
First blood specimen removed is used for laboratory tests.
Patient should be allowed to sit up after procedure and have a sugary drink (parents are asked to bring a sweet drink at each visit). If required 5-10ml/kg of normal saline can be administered.
After 15-30 minutes patient may be discharged if well.
Precautions explained to child/parent prior to each discharge:
- Keep activity to a minimum on same day (e.g. no active sports) especially if returning to school
- If feeling faint (cold, clammy) within few hours after venesection:
- sit down and put head between knees or lie flat
- have a sweet drink if possible
- stay sitting/lying until feeling better
- slowly get back onto feet
- Take it easy for rest of day.
- Inform ANP or DCU of fainting episode or unwell after discharge.
For further information contact:
On-call haematologist
Franchini M, Gandini G et al 2004. Efficacy and safety to reduce transfusional iron overload in adult, long-term survivors of acute leukaemia. Transfusion 44: 833-837
Angelucci E et al, 1997. Phlebotomy to Reduce Iron Overload in Patients Cured of Thalassemia by Bone Marrow Transplantation. Blood 90(3): 994-998
Sucak G et al, 2012. The prognostic role of hemochromatosis H63D allele in allogeneic hematopoietic stem cell transplantation. Ann Hematol 91: 1281-1287
Majhail N et al, 2010. A Prospective Study of Iron Overload Management in Allogeneic Hematopoietic Cell Transplantation Survivors. Biol Blood Marrow Transplant 16: 832-837
Fred Hutch & Seattle Cancer Care Alliance. Information for physicians: resources for physicians treating LTFU patients.
PDWP Management of Iron Overload in Stem Cell Transplant 2019 - Nava T et al. Supportive care during pediatric hematopoietic stem cell transplantation: beyond infectious diseases. A report from workshops on supportive care of the Pediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 2020.
Last reviewed: 21 June 2023
Next review: 30 June 2025
Author(s): F Pinto
Version: 6
Approved By: Schiehallion Clinical Governance Group
Document Id: RHC-HAEM-ONC-010

