Acute wheeze: escalation to intravenous therapy for Acute Wheeze Integrated Care Pathway (ICP) for patients over 2 years old
Objectives
Standardisation of the management of children over 2 years of age requiring intravenous (IV) therapy for management of acute wheeze.
Scope
Children aged 2-16 years presenting with severe or life threatening wheeze as per BTS/SIGN criteria and have failed to respond to initial therapy. Children under the age of 2 years should follow the separate guideline for children under the age of 2 years with wheeze.
Audience
Medical and nursing staff who encounter this patient group
November 2023: This guidance is currently under review as it has gone beyond the standard review date. It reflects best practice at the time of authorship / last review and remains safe for use. If there are any concerns regarding the content then please consult with senior clinical staff to confirm.
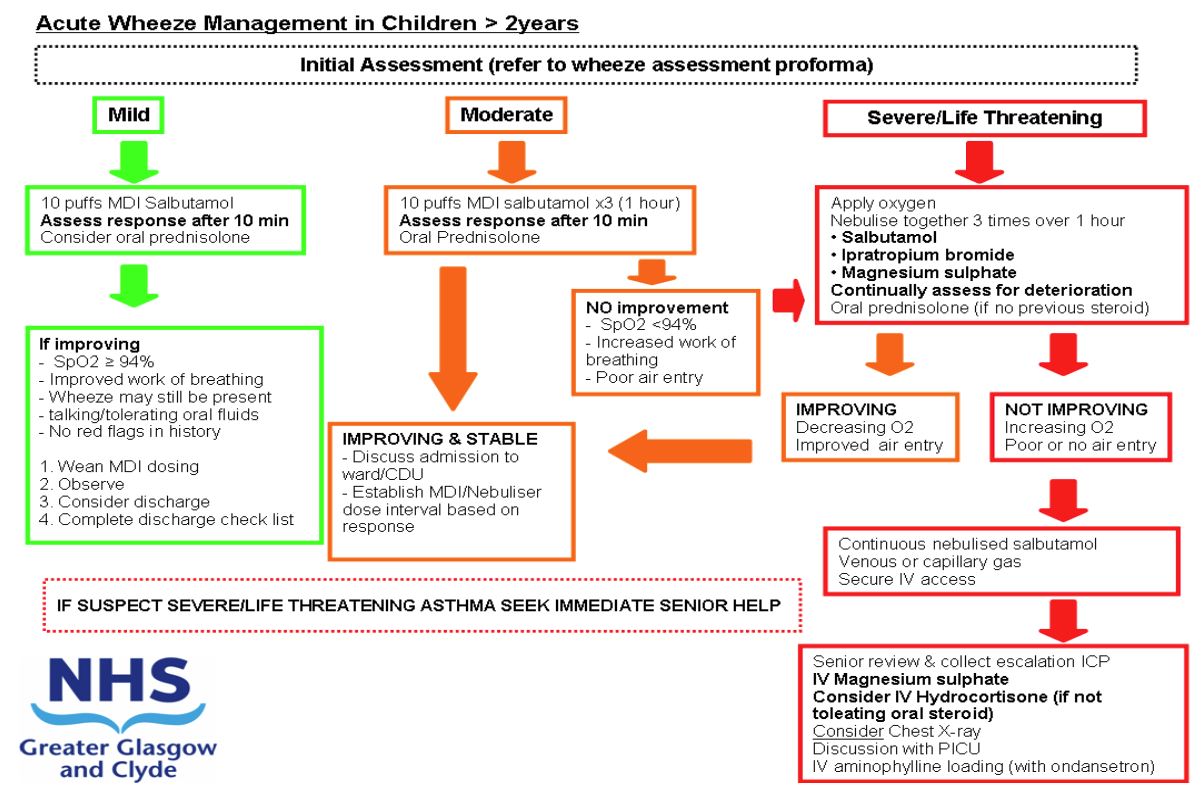
Wheeze severity assessment
|
MILD AND
|
MODERATE AND
|
|
SEVERE AND
|
LIFE THREATENING AND
|
'Red Flag features
- Has the patient previously received IV therapy for wheeze management?
- Has the patient been admitted to the PICU previously for respiratory illness?
If YES to any of the above then patient should be discussed with on call Paediatric Registrar prior to discharge.
Drugs
Salbutamol MDI + Spacer – Initial therapy = 10 puffs. (100mcg per puff)
Oxygen – minimum 6 l/min via non-rebreather mask
| Prednisolone |
|
2 -4yrs 20mg OD >5yrs 40mg OD |
| Nebulised medication for Severe Wheeze | |
|
2 -4yrs |
Salbutamol 2.5mg |
| >5yrs |
Salbutamol 5mg |
IV MEDICATION
(To be prescribed as per the Escalation to IV therapy care pathway)
| 1. Magnesium sulphate injection |
40mg/kg over 20 minutes (max 2gram) |
||||
|
2. Aminophylline |
|
||||
|
3. Salbutamol |
|
||||
|
- Hydrocortisone - Ondansetron |
4mg/kg QDS (max 100mg) 100micrograms/kg (max 4mg) |
Discharge criteria & checklist
- Patient maintaining saturations > 94% in air
- Tolerating 3hrly multidosing
|
Patients with MILD asthma at 1st assessment can be discharged after Salbutamol without being monitored for 4 hours |
- Discharge Checklist Completed
- No red flag features
- If presenting with interval symptoms medication reviewed and consideration given to starting Clenil Modulite 100mcg BD
- Follow-Up arranged as below
|
DISCHARGE PLANNING – POINTS TO CONSIDER |
Criteria for Acute Medical Paediatric Follow Up |
| Discharge Checklist Completed? All the following must be completed prior to discharge
|
GP’s should be able to manage the majority of children with wheeze Patients who have been started on a clenil inhaler should be advised to attend their G.P. in 6 weeks to assess response. Children where there is diagnostic uncertainty or very young children (between the ages of 2 and 3) with concern about recurrent presentations then discuss Follow up planning with either the general paediatrician or senior paediatric registrar on tel: 84678. |
|
CHRONIC FEATURES / INTERVAL SYMPTOMS |
Criteria for Respiratory Team Follow Up |
|
If any of the following features:
AND not on a Preventer inhaler then prescribe Clenil modulite 100mcg BD If already on a preventive inhaler review compliance and |
Any child requiring intravenous therapy for wheeze. Patients who have required intravenous therapy for wheeze should be monitored in hospital for at least 24 hours post the discontinuation of all intravenous therapy. Any child where there is a concern that they have failed to respond to significant asthma treatment. ALL RESPIRATORY REFERRALS SHOULD BE DISCUSSED WITH RESPIRATORY TEAM PRIOR TO PATIENT DISCHARGE FROM HOSPITAL. |
- Failure to respond to initial therapy as per GGC ‘Acute wheeze’ guideline
- Assessing Response
- Consider Escalation to Intravenous Therapy
- Escalation to IV therapy – drug algorithms
- Magnesium Sulphate
- Aminophylline
- Salbutamol
- Monitoring – including drug levels – timing and actions to levels
- When to de-escalate care & referral to Respiratory Services criteria.
- Other care considerations – CXR and Virology sampling.
- Safe transfer criteria
- Estimated Weight Chart
|
Features of Life Threatening Asthma Oxygen saturations <92% in air AND Drowsy or confused Silent Chest and CO2 retention are features of life threatening asthma. *************** SEEK SENIOR HELP IMMEDIATELY *************** |
- Give high flow oxygen via tight fitting face mask to achieve saturations 94% or above
- Continuous monitoring of heart rate and oxygen saturation
- Continue nebulised Salbutamol therapy
- Ensure the child has received oral steroids
(if oral steroids not tolerated, see IV hydrocortisone below) - If a child is already on daily steroids increase the dose to 2mg/kg (maximum 60mg)
- Assess response to initial treatment
Please document history and examination in ‘Acute Wheeze pro-forma’
Does the child meet criteria for Severe or Life-threatening Asthma?
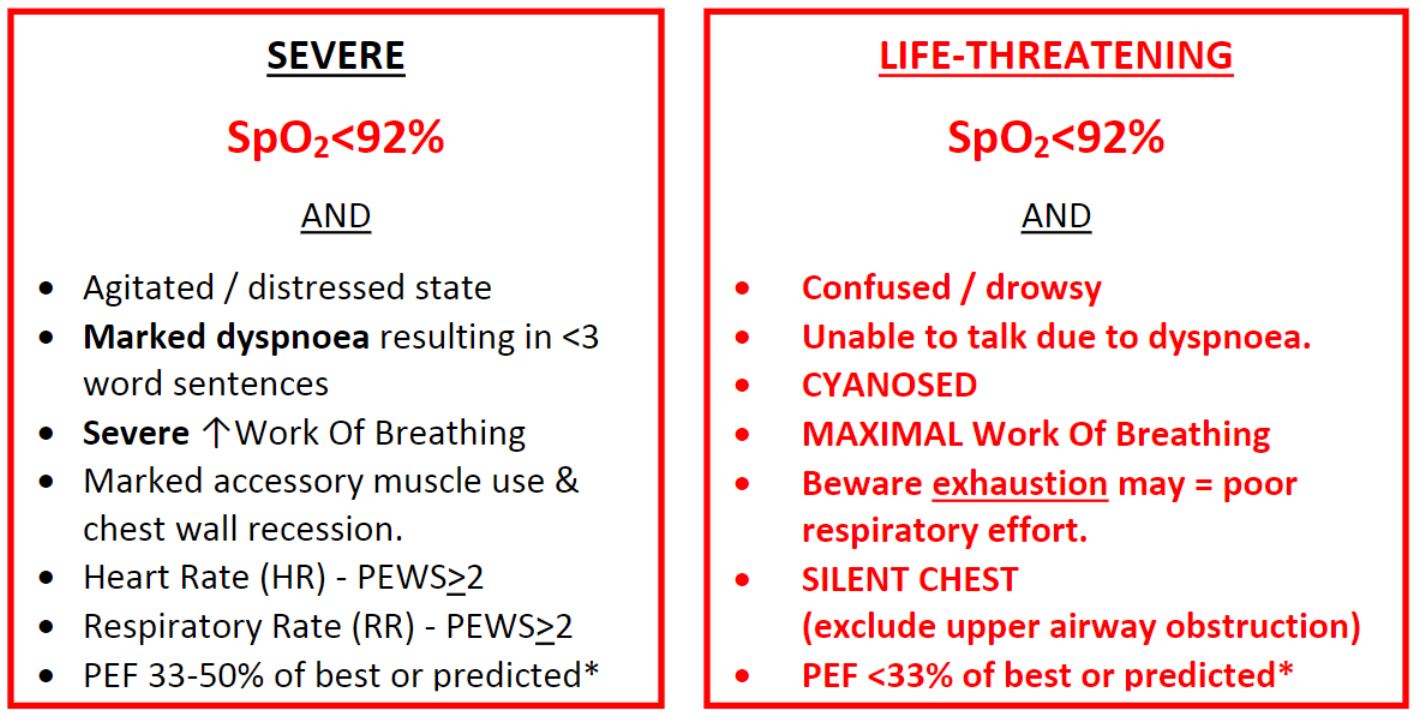
*Peak Expiratory Flow readings should be used in children who have previously used a Peak Flow meter. Falsely low readings can be obtained in patients not used to the technique
IF YES THEN CONTINUE WITH FOLLOWING GUIDANCE
|
Do they have features of LIFE THREATENING wheeze at presentation? If YES then commence nebulisers and proceed to IV therapies. |
DOES THIS PATIENT REQUIRE IV THERAPY FOR WHEEZE?
- Have they failed to show any improvement or deteriorated since their presentation (taking into account severity at presentation)?
- Do they have a significant or increasing oxygen requirement?
A small decrease in oxygen saturation is common after initial bronchodilator therapy and should be taken in the context of clinical condition and response to treatment.
Significant hypoxia is indicative of severe asthma
(SpO2 <92% in air, or >6l/min oxygen to maintain normal saturation) - Do they have increased work of breathing (severe or life threatening)?
- Do they have significantly reduced air entry or silent chest?
- Are there clinical signs of exhaustion?
|
If YES to ANY of the above obtain senior clinical review. |
RECORD CLINICAL PARAMETERS AND COMPLETE THE FOLLOWING:
- Heart rate / Respiratory rate / Conscious level / Blood Pressure
- Oxygen saturation (And note oxygen requirement in L/min)
- Continue with oxygen delivered nebulised therapies.
- Site IV cannula and take a blood gas and U&Es.
CO2 > 6kPa on venous gas is likely to indicate CO2 retention.
(if IV access challenging – CBG can be utilised as 1st line assessment). - Patients requiring intravenous infusions for management asthma should have continuous cardiac monitoring (3 lead)
- If oral steroids were not tolerated give IV hydrocortisone as per weight (weight unknown use table below) and prescribe - 6 hourly dosing.
|
Age |
Hydrocortisone Dose |
|
2-5 years |
50mg QDS |
|
5-16 years |
100mg QDS |
|
Raised lactate (>4) can be indicative of impaired oxygen delivery (due to poor gas exchange) or excess beta-adrenergic stimulation causing metabolic acidosis. It should be taken in the context of other clinical observations |
1. Magnesium Sulphate (MgSO4)
Drug dose
The dose is 40mg/kg or 0.16mmols/kg (max. 2 grams)
Drug Preparation
Draw up 0.08mls/kg 50% Magnesium Sulphate (40mg/kg) and dilute to 50mls with 0.9% saline
Run over 20-30 minutes.
|
ASSESS RESPONSE
If response to intravenous magnesium sulphate is felt to be poor, document the clinical parameters and relevant examination findings.
Then proceed to 2nd IV therapy – Aminophylline
|
Patients requiring AMINOPHYLLINE have severe/life threatening asthma. ENSURE THE MOST SENIOR CLINICIAN IN YOUR DEPARTMENT IS AWARE WHEN COMMENCING. |
2. IV AMINOPHYLLINE
Drug dose
The LOADING dose of aminophylline is 5mg/kg
(5mls/kg of 1mg/ml solution) over 20 minutes (Maximum dose 500mg).
Aminophylline LOADING should be prescribed using actual body weight unless patient is obese then use ideal body weight (see end of document)
Patients who are on theophylline should have a level taken and should not receive a loading dose.
All patients being given IV Aminophylline must be on continuous cardiac monitoring.
Drug preparation
Add 20mls of aminophylline 25mg/ml to 480mls of 0.9% sodium chloride to give a concentration of 1mg/ml.
Aminophylline is compatible with I.V. fluids which contain potassium; maintenance I.V. fluids can be connected via a y-connector.
ASSESS RESPONSE
Clinical review essential post LOADING to assess need for ongoing INFUSION.
Document clinical parameters and relevant examination findings.
|
If ongoing need for INFUSION, inform PICU to ensure patient is added to the 'Watcher list' - PICU Fellow: 84725 |
INFUSION Dose: ideal body weight for height (See BNFc for guidance chart).

Drug preparation
As per loading dose drug preparation above.
|
IF PATIENT HAS LIFE THREATENING ASTHMA / NOT RESPONDING TO AMINOPHYLLINE INFUSION THEN PROCEED WITH IV SALBUTAMOL. IV SALBUTAMOL CANNOT BE RUN CONCURRENTLY WITH IV AMINOPHYLLINE. SEPARATE CANNULAS MUST BE SITED TO RUN MULTIPLE IV THERAPIES CONCURRENTLY |
(See section below for details on guidance on monitoring requirements and clinical management of patients on Aminophylline infusion)
IV SALBUTAMOL
Salbutamol for injection comes in 2 concentrations:
500 micrograms in 1ml or 5mg in 5ml (1mg/ml).
Use the correct concentration for LOADING and INFUSION dosing.
|
LOADING DOSE Drug dose The LOADING dose of salbutamol is 15 micrograms/kg (maximum 250 micrograms) slow injection over 5-10 minutes. Drug Preparation Use 500 micrograms in 1ml concentration for LOADING preparation For intravenous injection dilute to a concentration of 50microgram/ml with 0.9% NaCl by adding 9mls to 1ml of 500microgram/ml salbutamol injection Example For any child over 16kgs then loading dose is maximum 250 micrograms 15kg child – LOADING dose = 15 x15mg = 225micrograms – |
|
INFUSION DOSE Drug dose INFUSION dose for salbutamol is 1-5micrograms/kg/min. Rates should be adjusted according to response. In the Royal Hospital for Children, IV salbutamol is a PICU LEVEL DRUG, infusions can be started pending transfer but doses of >2micrograms/kg/minute should not be given outside PICU. Drug Preparation Use 5mg in 5ml (1mg/ml) concentration for INFUSION preparation Example 20kg child – infusion dose is 2microgram/kg/min =40microgram/kg/min |
If IV SALBUTAMOL required then Treating Consultant should be present and PICU contacted asking for URGENT REVIEW (84725).
Children with severe asthma requiring intravenous therapy are high dependency (HDU) patients requiring close monitoring. Under our current pathway these patients would initially be admitted to CDU or PICU.
Continuous ECG and saturation monitoring is necessary for patients on aminophylline and/or salbutamol infusions.
- Standard clinical observations should be recorded on PEWS chart every 5 minutes for the first 15 mins and if improving then at 15 minutely intervals for the first hour.
- Observations, including BP should be recorded hourly until the patient is felt to be improving.
- CBG /VBG should be repeated as clinically indicated.
- U&Es should be conducted minimum of every 12 hours for patients on IV infusions.
CLINICAL REVIEW REQUIREMENTS
After starting an intravenous infusion the child should be reviewed (by physician or ANP) a minimum of every 30 minutes for the first 2 hours and as clinically indicated thereafter.
The following should be recorded in medical notes with each review:
- Conscious Level.
- Oxygen saturations and oxygen requirement in litres/minute.
Significant hypoxia (SpO2 <92%, or significant oxygen requirement >6l/min to maintain normal oxygen saturation) is indicative of severe asthma. - Heart rate – Compare to normal range for age.
Increasing tachycardia generally denotes worsening asthma but remember that B2agonists increase heart rate.
In life-threatening asthma a drop in heart rate can be a pre-terminal sign - Blood Pressure
- Respiratory Rate – Compare to normal range for age.
- Posture / position of patient.
- Ability to talk in words, phrases or sentences
- Degree of Respiratory Distress / Use of accessory muscle and recession
- Air entry - including any clinical suspicion of pneumothorax or significant collapse and amount of wheezing including biphasic or silent chest
Example of expected format of documentation of clinical review:
“Alert and orientated with saturations of 95% in 5L O2.
HR: 125 beats per minutes. RR: 32 breaths per minute.
Sitting forwards and speaking in short sentences.
Moderate subcostal and intercostal recession with tracheal tug.
Air entry is reduced bilaterally at the bases with biphasic wheeze throughout.”
Possible Side Effects of Intravenous Aminophylline
- Muscle tremors
- Tachycardia and palpitations
- Nausea and vomiting
- Agitation
- High doses can cause peripheral vasodilatation which can result in hypotension. Please see guidance for aminophylline in obese patients.
|
If a child develops a significant metabolic or lactic acidosis, without an increase in respiratory effort or oxygen requirement check an aminophylline level and adjust the infusion. |
Indication for taking Aminophylline Levels
The BNFc recommends aiming for theophylline levels of 10-20mg/L. There is very little evidence for this range in children3
For children in the RHC, Glasgow; we do not recommend routine theophylline levels for patients on aminophylline infusions.
|
Theophylline levels should be taken in patients who are on oral theophylline prior to starting aminophylline infusion or after 4 hours in patients who:
|
In these circumstances a level should be taken and sent to biochemistry urgently. These patients require a senior review.
DOSE ADJUSTMENT GUIDANCE:
POOR RESPONSE but no concern about toxicity:
< 5mg/L
Check the prescription and cannula site.
Repeat loading dose and leave the infusion running at the same rate. After this adjustment, if the patient is improving there is no need to check a further level.
5-<10mg/L
Repeat half-loading dose and leave the infusion running at the same rate. After this adjustment, if the patient is improving there is no need to check a further level
10-<12.5mg/L
Increase the infusion rate by 20%. After this adjustment, if the patient is improving and there are no signs of toxicity there is no need to check a further level
12.5mg/L -17.5mg/L
Increase the infusion rate by 10%. After this adjustment, if the patient is improving there is no need to check a further level.
> 17.5mg/L
Continue aminophylline infusion at the same rate. Urgent PICU review for consideration of intravenous salbutamol
Concern patient demonstrating signs of TOXICITY:
>20mg/L and clinical signs of toxicity (see above)
Withhold infusion for 4 hours and recheck level before restarting infusion; if intravenous therapy still required urgent PICU review for consideration of intravenous salbutamol
|
In all instances, failure of an adequate response to aminophylline requires urgent PICU review and consideration of intravenous salbutamol (irrespective of the aminophylline level) |
Remember that if a child is improving care can be de-escalated at any time
(Note: The half-life of aminophylline is 3-5 hours)
Criteria for reducing aminophylline:
- Normal ability to speak
- No increase in respiratory effort
- Reduction in oxygen requirement
- Significant improvement in peak flow
- Aminophylline rate should be halved and reviewed again for discontinuation after 6 hours
Some patients may require a slower reduction and aminophylline rate should then be halved every 6 hours. - The decision to reduce and discontinue aminophylline should be clearly documented in the notes. The change in rate and discontinuation of aminophylline needs to be documented (with timing) on the infusion chart.
|
Rebound bronchospasm can occur as aminophylline is reduced and stopped. Patients who have required intravenous therapy for wheeze should be monitored in hospital for at least 24 hours post the discontinuation of all intravenous therapy. |
All patients requiring intravenous therapy for wheeze should be referred as an in-patient to the respiratory team and the asthma specialist nurse (via TrakCare) in order for appropriate follow-up to be arranged.
Chest X-Ray
Chest x-rays are not routinely recommended in asthma.
If there is clinical concern about a pneumothorax or doubt about the diagnosis; a chest x-ray should be performed.
Virology
Episodes of wheeze are often triggered by respiratory viruses. Although a viral throat swab is not necessary during the acute resuscitation, it is helpful for children requiring intravenous therapy to have a viral throat swab taken as soon as clinically able.
Safe transfer criteria
Patient should not be transferred between clinical areas until clinically stable or deemed appropriate by ED/Gen Paeds/PICU consultant.
|
If transferring between hospital on IV therapies:
|
|
Age |
Weight (kg) |
|
3 years |
14 |
|
5 years |
18 |
|
7 years |
23 |
|
10 years |
32 |
|
12 years |
39 |
|
14 year old boy 14 year old girl |
49 50 |
|
Adult male Adult female |
68 58 |
- BTS/SIGN British guideline on the management of Asthma; September 2016:
- Mechanism of lactic acidosis in children with acute severe asthma; Meert KL, McCaulley L, Sarnaik AP. Paediatric Critical Care Medicine. 2012 Jan;13(1):28-31
- A Clinical Guideline for the use of Aminophylline in Acute Severe Asthma in Children; Norfolk and Norwich University Trust. Dr Caroline Kavanagh; 5th April 2017
- Aminophylline Dosage in Children Asthma Exacerbations in Children: A Systematic Review; Cooney L, Sinha I, Hawcutt D. PLoS One. 2016.
- Standards for Level of Asthma Intervention; Greater Glasgow and Clyde Health Board
- Aminophylline Hydrate; December 2015.
- BNF for Children. Aminophylline.
- BNF for Children. Hydrocortisone
- BNF for Children. Prescribing for children: weight, height and gender.
- Clinical Practice Guidelines: Asthma Acute. The Royal Hospital for Children, Melbourne. May 2015
Last reviewed: 01 October 2019
Next review: 31 October 2024
Author(s): Dr Steve Foster (Consultant in Paediatric Emergency – Paediatric Emergency Department), Dr Morag Wilson (Consultant in General Paediatrics – Acute Paediatrics)
Version: 2
Approved By: Paediatric Emergency Department Guidelines Group
Document Id: 1128