Adrenaline auto injector prescription guideline
exp date isn't null, but text field is
Objectives
The document provides guidance on the prescription of adrenaline auto injectors (AAI) for children.
Scope
The guidance should be used by healthcare professionals managing children at risk of severe allergic reactions/anaphylaxis.
The prescription of an adrenaline auto injector should be on the basis of a risk assessment which indicates a significant risk of anaphylaxis. This should only be considered;
- As part of a full allergy assessment including identification of triggers, education on avoidance, assessment of risk and the provision of an allergy management plan or whilst a full assessment is awaited if risk deemed considerable
- Where adequate training in appropriate usage has been given
- Where referral or follow-up has been arranged and where rationale for prescription can be reviewed and/or training re-enforced
The British Society of Allergy and Clinical Immunology has published a detailed guideline for prescribing an AAI and it is available on line for further information (Adrenaline Auto-Injector - BSACI).
Mild to moderate allergic reaction is characterized by one or more symptoms or signs of skin and/or gastrointestinal tract involvement without respiratory and/or cardiovascular involvement.
Anaphylaxis is a serious systemic hypersensitivity reaction that is usually rapid in onset and may cause death. Severe anaphylaxis is characterized by potentially life-threatening compromise in airway, breathing and/or the circulation, and may occur without typical skin features or circulatory shock being present.
Absolute indications for AAI:
- Previous anaphylaxis* triggered by food, latex or aeroallergen
- Previous exercise-induced anaphylaxis
- Previous idiopathic anaphylaxis
- Co-existing unstable or moderate-to-severe persistent asthma and a food allergy*
- Hymenoptera venom (bee and wasp stings) allergy in untreated patients (not receiving immunotherapy) with more than cutaneous/mucosal systemic reactions or high risk of re-exposure.
*Excluding oral allergy syndrome (OAS or pollen food allergy syndrome) unless patient has previously experienced systemic symptoms. The OAS is generally considered to be a mild form of food allergy; mild irritant symptoms such as itching of the mouth, lips and throat when eating raw fruits and vegetables.
Relative indications are (especially if more than one is present):
1) Previous mild-to-moderate allergic reaction to traces of food*
2) Previous mild-to-moderate allergic reaction* to foods known to be associated with anaphylaxis (e.g. peanut and/or tree nut, cow's milk etc.).
3) Teenager or young adult with a food allergy with previous mild-to-moderate reactions*
4) Remote from medical help or prolonged travel abroad in the context of previous mild-to-moderate allergic reactions to a food, hymenoptera venom (bee and wasp stings), latex or aeroallergens
*Excluding OAS unless patient has previously experienced systemic symptoms
Absolute indications
- During and after venom immunotherapy, in patients with more than cutaneous/mucosal systemic reactions if risk factors for relapse are present.
- Underlying systemic mastocytosis; children with very severe skin involvement (>50% body surface) and increased basal serum Tryptase levels (>20 ng/ml) and with blistering in the first three years of life.
Relative indications
- Oral immunotherapy for food allergy
- Hymenoptera venom or drug allergy in patients with more than cutaneous/mucosal systemic reactions and cardiovascular disease
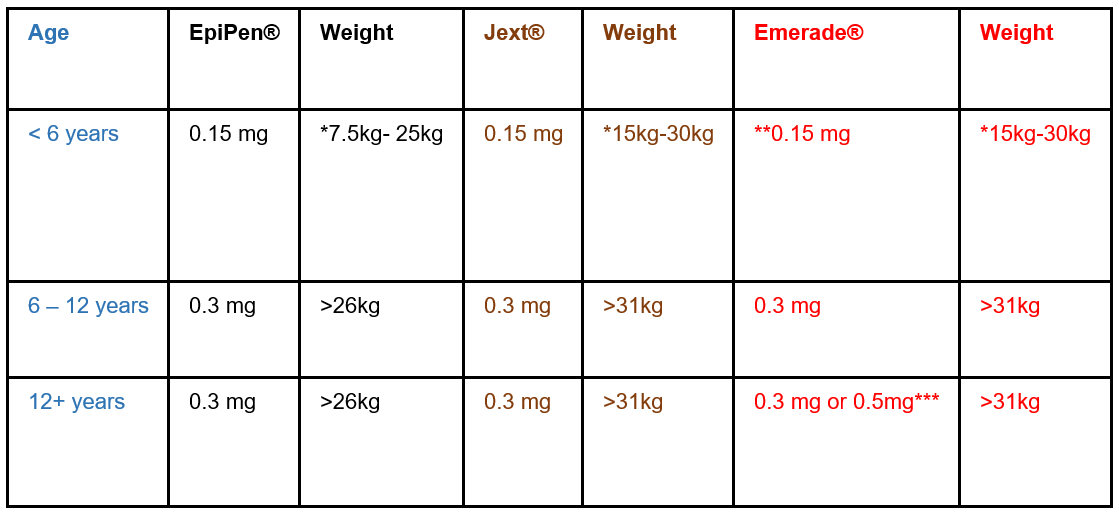
* Infants at HIGH risk of accidental exposure and who weigh more than 7.5 kg can be issued with a junior AAI (0.15mg). For infants less than 6 months of age, allergen avoidance measures should be effective in most cases.
** Product not available, see BNFc important Safety Information
*** if > 60 kg; if small or pre-pubertal offer 0.3 mg
Patients should be given a prescription for 4 devices; two in the emergency bag (giving the option of administering 2 doses) and two for the nursery/school.
- Emergency treatment of anaphylactic reactions: Guidelines for healthcare providers; Working Group of Resuscitation Council UK May 2021
- EAACI guidelines: Anaphylaxis (2021 update)
- World Allergy Organisation Anaphylaxis Committee. Anaphylaxis Guidance 2020
- Adrenaline Auto-Injector – British Society Allergy Clinical Immunology (2016)
- BNFc
Last reviewed: 01 November 2022
Next review: 30 November 2026
Author(s): George Raptis
Approved By: Stakeholders: Specialist Allergy Nurses; Medical Team; Pharmacy; Respiratory Team
Document Id: 142

