Cardiac genetics pathway for infants with congenital heart disease and the appropriate utilisation of irradiated blood
exp date isn't null, but text field is
Objectives
The aim of the pathway is:
- to specify which genetic investigations are required for children with congenital heart disease in Scotland.
- to specify further investigation of those with a micro-deletion of chromosome 22q11.
- To specify which patients need irradiated blood
- To facilitate input from Clinical Immunologists and Clinical Geneticists
- to minimise the inappropriate use of irradiated blood
This advice should be viewed in association with the guidance regarding Blood Product Requirements for Cardiac Surgery and Catheter lab procedures.
Scope
This pathway should be used by healthcare professionals involved in infants with congenital heart disease.
Some patients with congenital heart disease (CHD) may have an associated T-cell immunodeficiency. This applies particularly those with conotruncal and aortic arch defects or right aortic arch, with or without other dysmorphic features who may have a deletion of chromosome 22q11, and also to patients with CHARGE syndrome.
Those with a severe T-cell immunodeficiency and are at risk of developing “transfusion-associated-graft-versus-host-disease” if they receive non-irradiated blood, as a result of engrafting of donor lymphocytes present in the transfused blood. Therefore further investigations of T- cell function are required (see appendix 1) in children with a proven micro-deletion of 22q11 to assess whether they are or are not at risk of transfusion-associated-graft-versus-host disease.
There is thus a need for testing (often urgently) of newly referred patients with CHD in order to decide, among other aspects, their need for irradiated blood.
A protocol for the diagnosis of common genetic abnormalities, in particular 22q11 deletion, has been developed for patients with newly diagnosed congenital heart disease. This will include standardised blood sampling for genetic disorders. A single sample of 2-3 ml of blood is taken for:
- Multiplex Ligation-dependent Probe Amplification (MLPA)
- rapid testing for 22q11 deletion syndrome (or DiGeorge Syndrome) and other micro-deletions including 10p14, 4q35, 8p22 and 17q13.
- results of 22q11 screening available in 2 to 3 working days.
- Single Nucleotide Polymorphism Array (SNP Array)
- more detailed chromosome testing which, unlike MLPA, allows the identification of micro-deletions (such as 22q11) or micro-duplications across the genome.
- disadvantage: it may identify small chromosome changes of uncertain clinical significance. Parental testing can help clarify the significance in some cases. This should be discussed when consent is obtained for genetic testing.
- results available in 14 days for those marked as urgent.
In addition recommendations are given for the testing of immune function in those found to have 22q11 microdeletion (Appendix 1).
Consultation with Clinical Genetics is always indicated for those patients found to have a genetic abnormality and also with Clinical Immunology for all patients with 22q11 deletion. Contact details are given in appendix 4.
Consultation with Clinical Genetics is always indicated for those patients found to have a genetic abnormality and also with Clinical Immunology for all patients with 22q11 deletion. Contact details are given in appendix 4.
The use of this pathway will be audited after a period of three years and the pathway amended if necessary.
Many patients may have undergone some form of antenatal genetic testing
- If only a fetal antenatal 22q11 test was undertaken then a postnatal SNP array should be undertaken as this could identify another cause for the CHD, other than 22q11 deletion
- If only a fetal antenatal SNP or micro-array was undertaken and is normal the patient does not technically need it repeated however it should be remembered the result will be under maternal CHI
- If the fetal antenatal array was abnormal then it will require postnatal validation in case of a mosaic result or other diagnoses
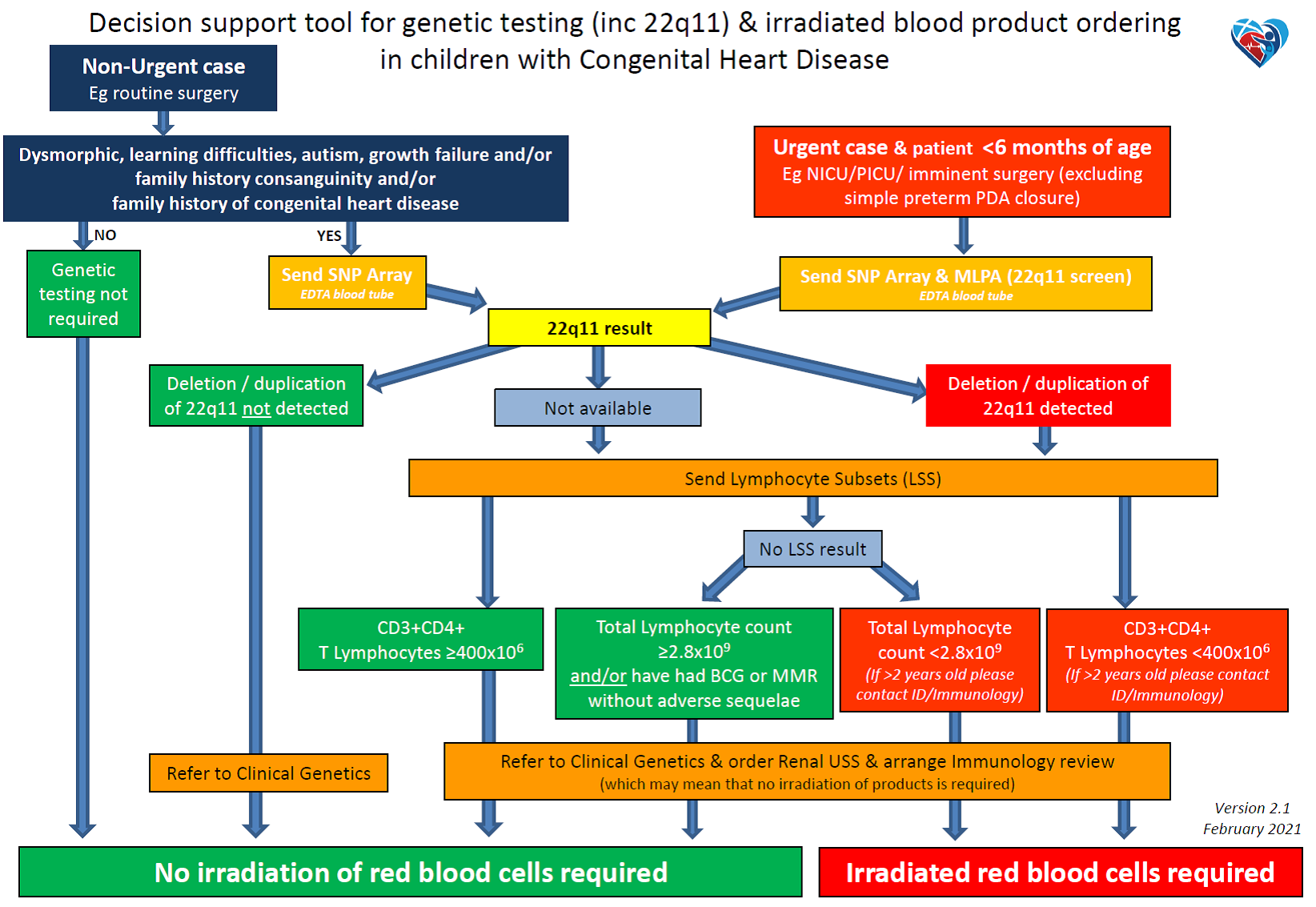
Urgent cases & less than 6 months old
All infants presenting to Cardiology, PICU, NICU or remote neonatal units with structural congenital heart disease or those for whom imminent surgery is planned should have the following genetic investigations undertaken after obtaining appropriate consent:
- Blood sampling
- 2-3ml blood in EDTA tube
- SNP Array
- MLPA (22q11 screen)
- 2-3ml blood in EDTA tube
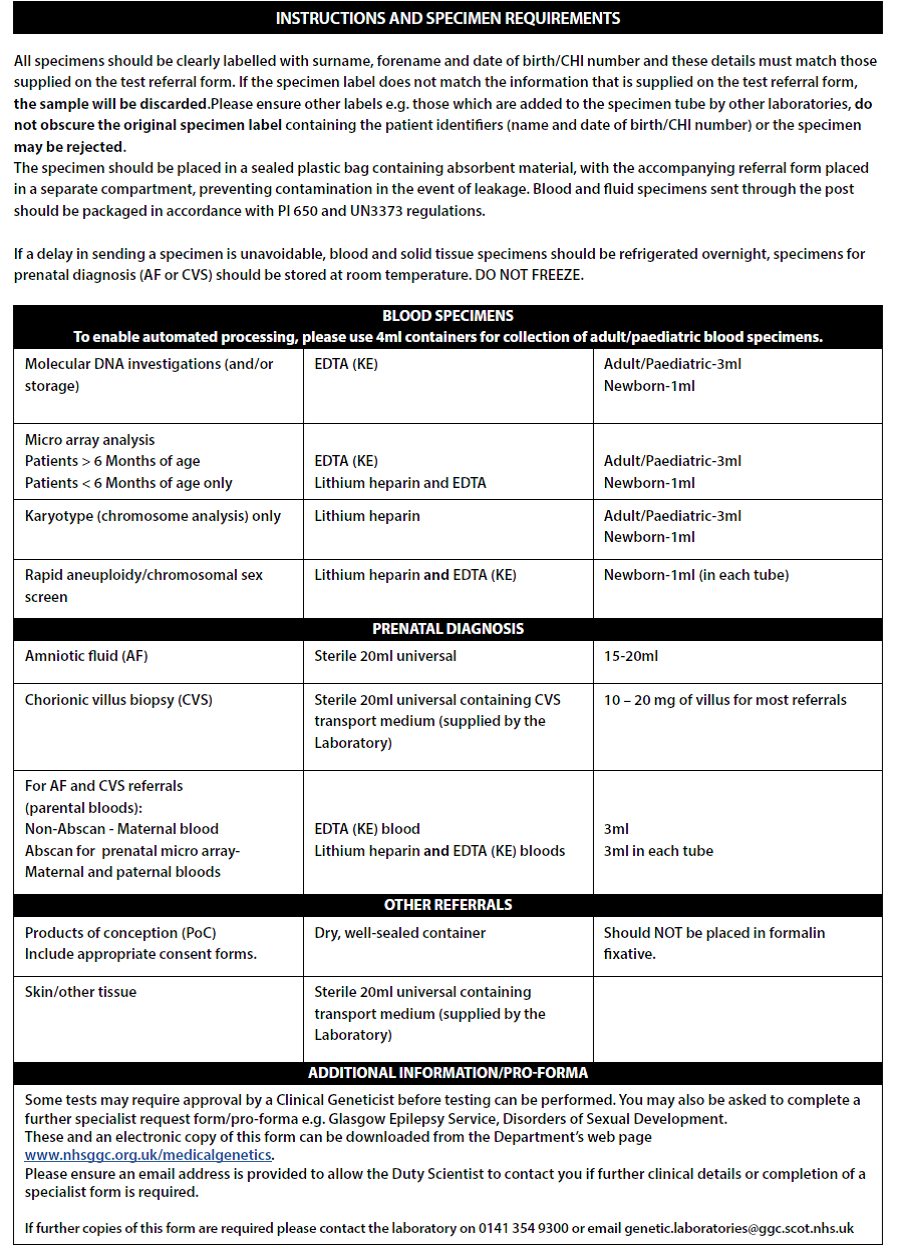
- Please complete request form (See image below – Full form in Appendix 3):
- Complete reason for referral & sample details
- In additional information write: Urgent Testing for MLPA (22q11 screen) & SNP Array
- If the child is going for surgery imminently please state the date of surgery
- Blood will routinely be stored for any potential subsequent genetic testing
- Consent
- As DNA will routinely be stored, ideally written consent should be obtained from the child’s parents. It is not essential to get consent for critical pre-operative investigations, such as MLPA for 22q11 screening. The standard proforma for genetic investigation has been adapted for this purpose. The consent form should be completed and stored in the clinical notes. (Appendix 2)
- Please note some results from SNP Array testing may require samples from both parents to aid the interpretation of any variations found.
Further genetic investigation will be guided following consultant review and/or review by clinical genetics.
Non-urgent cases
All children with congenital heart disease for whom testing is not urgent and in whom there is a possible genetic cause – i.e. presence of a cardiac anomaly as well as learning difficulties, autism, abnormal growth pattern, dysmorphic features, associated congenital malformation(s) of other organs or a family history of 22q11 deletion, velocardiofacial syndrome, diGeorge syndrome, consanguinity or cardiac /arch anomaly should be referred to the Clinical Genetics team. They should also have the following samples collected for SNP Array and stored for subsequent analysis ensuring the clinical features are noted on the request form. Appropriate consent should be obtained (see appendix 2).
- Blood sampling
- 2-3ml blood in EDTA tube
- SNP Array (see below)
- 2-3ml blood in EDTA tube
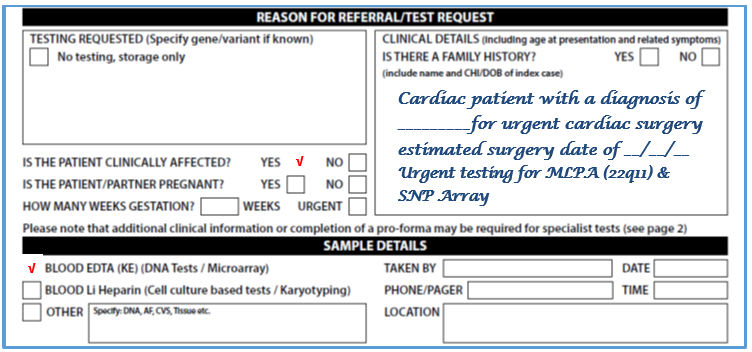
- Please complete request form (See image below – for full form see Appendix 3)
- Select DNA analysis box
- In additional information write: Testing for SNP Array
- Blood will routinely be stored for any potential subsequent genetic testing
- Consent
- As DNA will routinely be stored, ideally written consent should be obtained from the child’s parents. The standard proforma for genetic investigation has been adapted for this purpose. The consent form should be completed and stored in the clinical notes. (Appendix 2)
Further genetic investigation will be guided following Clinical Genetics Consultant review.
CLINICAL GENETICS REVIEW
- Patients with congenital heart disease and other features suggestive of a genetic diagnosis will be offered a review by the Clinical Genetics team.
- The Duty Clinical Geneticist can be contacted through the Clinical Genetics Reception on 0141 354 9201. Leave the relevant patient details and please state how quickly you would like the baby to be seen.
- Where appropriate, the Clinical Genetics team will advise on appropriate further investigations, for which consent must be obtained. For the majority of cases it is expected that no further samples will be required.
- The Paediatric Cardiac Link Medical Genetics Consultant is Dr Ruth McGowan who can be contacted on: Telephone: 0141 354 9246 Email: ruth.mcgowan@ggc.scot.nhs.uk
RESULTS OF GENETICS INVESTIGATIONS
- Positive results of urgent genetic investigations will be telephoned to the relevant unit by the genetics laboratory staff who will also inform the on-duty Clinical Geneticist. Positive results should be documented clearly in the clinical notes.
- Urgent samples will be reported as soon as possible, with 22q11 results being available within 2-3 working days.
- Results of all tests are now available on the Clinical Portal.
- The patient’s parent or carer should be informed by a senior member of staff at the earliest opportunity. The patient’s GP will be informed through the discharge summary and by direct communication from the Clinical Genetics team in due course.
- It is the responsibility of the patient’s medical Consultant (Intensivist, Cardiologist or Neonatologist) to ensure that Blood Bank and the Infectious Diseases & Immunology team are notified when a 22q11 micro-deletion is detected (See sections 5 & 6).
- Likewise, it is the responsibility of the patient’s clinical team to inform Blood Bank if irradiated blood is no longer needed, for example:
- urgent blood was irradiated for a patient who is subsequently proven not to have 22q11 deletion
- a patient with proven 22q11 deletion is found to have no T-cell immunodeficiency and therefore no longer requires irradiated blood.
CHARGE syndrome
If features are suggestive of CHARGE (Coloboma, Heart anomaly, Atresia choanae, Growth retardation and Ear anomaly) syndrome then please discuss with immunology and clinical genetics teams. The advice detailed below also applies to patients with CHARGE. Please note that if this diagnosis is suspected then other specific genetic testing (e.g. CHD7 testing) may be appropriate.
Any child found to have a 22q11 micro-deletion should have lymphocyte subsets sent for quantitative assessment of lymphocyte T-cell numbers prior to any blood product transfusion, if possible. Results are available within 2–3 working days.
All patients with 22q11 micro-deletion should be discussed with the duty Infectious Diseases & Immunology Consultant as soon as possible, with a view to arranging review after lymphocyte subset results are available.
Red blood cells
Irradiation inactivates donor lymphocytes and is the only known method of preventing “Transfusion-Associated-Graft-versus-Host Disease” (TaGvHD), a rare but potentially fatal complication of blood transfusion.
If transfusion might be necessary for cardiac or other interventional or surgical procedures, blood must be irradiated and be passed as CMV-negative before being given to the following three groups:
- All patients with known 22q11 deletion and/or T-cell immunodeficiency
- All patients requiring urgent surgery with known 22q11 deletion but whose immune function is not yet known
- All patients requiring urgent surgery whose 22q11 and immune function status is not yet known
As with older children, the need for irradiated blood should be discussed with the immunologist if there is a question regarding their need for irradiated blood.
CMV-negative blood products should be given to all patients up to 28 days of age or until they reach 28 days post their estimated date of delivery (EDD) whichever is the latest.
The provision of CMV-negative, irradiated blood is resource- intensive and costly, prolongs the preparation time and leads to increased blood wastage. The local Blood Transfusion Service (BTS) provides guidance1 regarding the appropriate utilisation of irradiated blood. Despite this, under current practice, many patients who do not have the T-Cell immune-deficiency associated with 22q11 deletion unnecessarily receive irradiated blood.
Other blood products
As platelets are routinely irradiated irrespective of immune function status of the recipient and as fresh frozen plasma and plasma products (e.g. anti-D, Albumin, immunoglobulin) do not need to be irradiated, consideration of irradiation applies only to blood itself, both fresh and packed cells.
NOTE: irradiated blood products must be used within 24 hours of irradiation.1
- Cardiology
All patients will have cardiology follow-up arranged prior to discharge.
- Genetics
All patients with a confirmed genetic diagnosis, ongoing investigation or awaiting results will be offered genetics follow-up.
- Immunology
All patients with a 22q11 deletion will have immunology review and follow-up arranged prior to discharge.

If you would like a baby seen urgently i.e. within 24-48 hours, please phone the Medical Genetics Reception on 0141 354 9201 and ask for the Duty Consultant Geneticist. Leave the relevant patient details and please state how quickly you would like the baby to be seen.
The Paediatric Cardiac Link Medical Genetics Consultant is Dr Ruth McGowan who can be contacted on:
Telephone: 0141 354 9246
Email: ruthmcgowan@ggc.scot.nhs.uk
Relevant contact details:
|
Greater Glasgow & Clyde |
All enquiries: 0141 354 9300 |
|
NHS Lothian |
Clinical Genetics: 0131 537 1061 / 1116 |
|
NHS Grampian |
Clinical Genetics: 01224 552120 |
|
NHS Tayside |
This form should be completed for all patients who have special requirements for blood components. A copy should be sent to Blood Bank and a copy filed at the front of the patients clinical notes. It is the responsibility of clinicians to update Blood Bank on any changes to special requirements. A minimum annual review is required.

-
Greater Glasgow and Clyde Blood Transfusion Special Requirements Policy.
www.staffnet.ggc.scot.nhs.uk/Acute/Diagnostics/BloodTransfusion/Pages/BloodTransfusion.aspx
Useful links (Staffnet links - access only from devices connected to the NHSGGC Network):
- West of Scotland Genetics Services
www.staffnet.ggc.scot.nhs.uk/Acute/Diagnostics/All%20Laboratory%20medicine/medical%20genetics/Pages/default.aspx - NHS GG&C Blood Transfusion Service
www.staffnet.ggc.scot.nhs.uk/Acute/Diagnostics/BloodTransfusion/Pages/BloodTransfusion.aspx - Special Requirements of Blood Transfusion Laboratory Request Form http://www.staffnet.ggc.scot.nhs.uk/Acute/Diagnostics/BloodTransfusion/Documents/GG_C%20Transfusion%20Policies/Special%20Requirement%20guideline%20%20for%20Blood%20Products%20Final%20-%20070313.doc
Last reviewed: 31 May 2021
Next review: 01 April 2024
Author(s): R Hague, AM Heuchan, M Campbell, B Knight, A Goldie, R McGowan, E Chalmers, C McKie, E Hanlon, M Davidson
Version: 2.1
Approved By: PICU & Cardiac Guideline groups
Reviewer Name(s): Responsible Clinical Directors: M Davidson & N Spenceley