Diabetes insipidus diagnosis and management (Including fluid management in children following suprasellar tumour surgery)
exp date isn't null, but text field is
Objectives
Children with a suprasellar tumours are at risk of developing panhypopituitarism, along with diabetes insipidus. This guideline has been written to aid in the diagnosis, post-operative management, monitoring and potential complications of diabetes insipidus. It also includes an algorithm for the management of a high urine output and a four hourly fluid balance chart.
Scope
Children with suprasellar tumours with the features of diabetes insidipidus.
Audience
Healthcare professionals involved in the care of neurosurgical patients.
Children with suprasellar tumours, particularly craniopharyngiomas, are at risk of developing panhypopituitarism together with diabetes inspidus. Some of these children may have symptoms at diagnosis. Baseline investigations may not always demonstrate an endocrinopathy. The patient may have inadequate ACTH and cortisol secretion, which may mask DI. This may be unmasked once hydrocortisone (dexamethasone) is started, or worsen DI requiring a change of dose of DDAVP. Therefore close monitoring is required during this time.
The diagnosis of diabetes inspidus is based on: elevated plasma osmolality due to hypernatreamia AND inappropriately dilute urine.
Management is to maintain plasma Na+ in the normal range and prevent large fluctuations.
The management of postoperative craniopharyngioma should take account of the triphasic pattern of vasopressin or antidiuretic hormone (ADH) secretion. This is as follows:
- Days 1-2 postop – diabetes insipidus (DI)
- initial hypothalamic damage with ADH insufficiency,
- Days 2-8 postop – syndrome of inappropriate ADH secretion (SIADH)
- necrosis of the posterior pituitary, with a large release of ADH
- Day 5+ → - Diabetes insipidus (usually permanent)
- ADH deficiency once the necrotic phase is over
The severity and duration of the first 2 phases are variable and treatment with both fluids and DDAVP must be cautious.
Click here to download Diabetes insipidus 4 hourly balance sheet
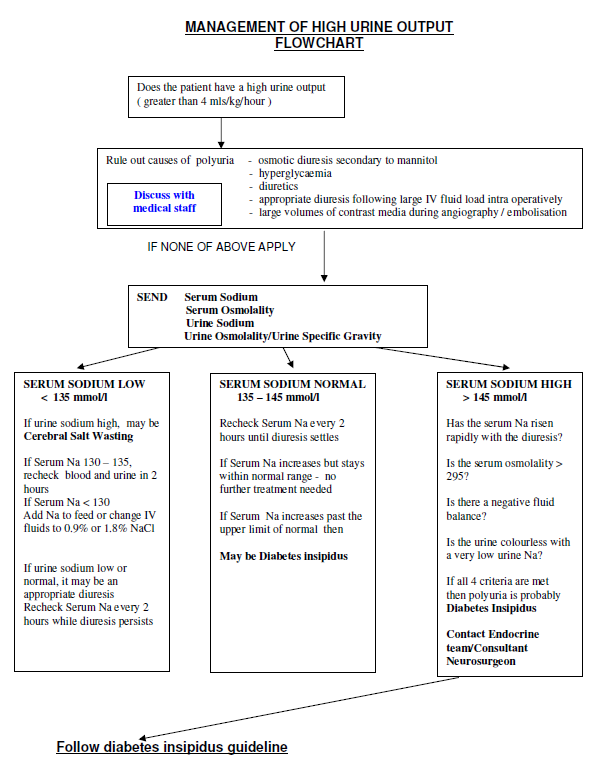
- Polyuria
- Polydipsia, but only if thirst centre is intact
- Dehydration/weight loss
A combination of the following (at least 3 out of 4):
- Urine output 5ml/kg/hour for 2 consecutive hours
- Plasma sodium > 145mmol/L
- Osmolality: plasma > 295 mOsmol/kg H2O And urine < 450 mOsmol/kg H2O
Urinary Specific Gravity (SG) < 1.005 (bedside test –same sample of urine as above) - Weight loss (if available) > 5% compared to a recent measurement
- Fluids 0.9% saline with dextrose . Hypotonic saline is viewed with extreme caution in neurosurgery and should only be used to treat a demonstrated hypernatraemia.
If catheterised:
Insensible losses (300ml/m2/day) – (See BNF for Children –back pages to work out m2)
+ estimated % dehydration replaced over 48 hr
+ previous hours urine output (either IV or orally if tolerating enteral fluids)
OR
If uncatheterised:
Maintenance fluids + estimated % dehydration replaced over 48 hrs. The maintenance fluid should then be reviewed twice daily and adjusted according to fluid balance, weight and U+Es. - Measure hourly urine output and specific gravity. ( 4hourly balance sheet)
(NB specific gravity can be crudely converted to osmolality by multiplying the last two digits by 40. For example urine SG of 1.005 = osmolality of 05 X 40 = 200 mOsm/Kg, SG 1015 = 15 x 40 =600 etc.) - If urine output exceeds 5ml/kg/hour and urine SG < 1.005 in the first few hours on return from theatre then DI is likely. If the patient is awake and able to drink with intact thirst, he should be allowed to drink freely and sodium should be monitored closely
Check plasma sodium and if 150mmol/l or greater and rising, discuss with endocrine team and consider a small stat dose of DDAVP. If in doubt it is safer to keep input/output in balance by allowing the patient to drink freely.
The aim is to avoid over-treatment resulting in rapid hyponatraemia, cerebral oedema and fits, therefore a conservative dose is initially given:
Subcutaneously – 0.04 microgram/kg (Maximum of 1 microgram)
Maximum dose can be increased to total dose of 2 micrograms following discussion with endocrine team.
1 month-2 years-400nanograms/(0.04mcg/kg)
2-12 years-0.5-1 microgram
12-18years-1-2 microgram
OR
Orally (these are available in 120 DDAVP melts microgram tablets)
Dissolve 120mcg in 12mls water, 1ml=10mcg
<2 years age 7.5 micrograms
>2 years age 15 micrograms
>10 years age 30 micrograms
If already on DDAVP, a dose of 25-50% of their usual dose may be needed.
Initially, these doses may need to be repeated every 4-24 hours if urine output does not normalise, as there is a wide variation in individual response. A breakthrough of urine output to pre-treatment levels should occur before another dose in given i.e.>5ml/kg/hr for 2 consecutive hours.
Click here to download Diabetes insipidus 4 hourly balance sheet
Measuring input/output 4 hrly, together with U+Es will prevent large Na+ fluctuations.
At least twice per day clinical and biochemical re-assessment is important to avoid over or under treatment. The goal is to be normovolaemic and “normonatraemic”. Hyponatraemia is more dangerous than hypernatraemia in post op patients because it can result in severe brain swelling and raised intracranial pressure. Therefore far better to under-correct than to overcorrect for DI.
- Strict fluid input and output-4 hourly balances
- Daily weight (8am and 8pm)
- Regular U+Es
First 12 hours post op- 2-4 hourly U+Es
4-6 hourly U+Es until day 3
Aim to keep Na+ 140-150mmol/ for first 48 hours
More frequent U+Es may be required if concerns re hypo/hypernatraemia
Hypernatraemia Na+>150mmol/l
increase the intravenous fluid intake and/or give a further dose of DDAVP. If Na+ is > 155-160 ensure that intravenous fluid is 0.9% saline
Sodium within normal range (135-145 mmol/l)
Do not give further DDAVP. Continue to monitor Na 4-6 hourly and fluid balance.
Caution patients may develop SIADH following a period of DI.
Hyponatraemia Na+<135 mmol/l
No further DDAVP should be given
The patient may have entered a period of SIADH and therefore consider:
If Na+<130mmol/l-Fluids should be restricted to 2/3rds of maintenance
Consider stat dose of frusemide 1mg/kg (IV or Orally)
If symptomatic/seizures, requires prompt treatment.
Give 4ml/kg of 3% saline over 15-20 mins and follow Hyponatraemia guideline
Avoid rapid changes in the plasma Na+
1. Over treatment with DDAVP
This will result in a strongly positive fluid balance, weight gain and rapidly falling plasma sodium levels and brain oedema. This is treated as follows:
- No further DDAVP should be given
- Fluids should be restricted to 2/3rds of maintenance
- If symptomatic, an infusion of 1.8% or 3% saline (1-2 ml/kg/hr of 3%, 0.5-1 mmol/kg over 2-3 hrs) may be required, which should raise the plasma sodium levels by 10mmol/L.
- 6 hourly U+E
- Eventually urine output will increase and sodium levels will correct. If this occurs too rapidly then fluid regime may have to be adjusted again. The aim is to correct the sodium by <12mmol/L per day
- DDAVP at a lower dose can then be re-introduced.
2. SIADH
Following neurosurgery (for suprasellar lesions) a period of DI (classically 4-8 days) may be followed by a period of high ADH secretion resulting in hyponatraemia due to free water retention and is accompanied by a normal urine output, low renin level and continued urine loss of sodium (>20mmol/L). This may last up to 2 weeks. This is treated as follows:
- no further DDAVP should be given
- Fluids should be restricted to 2/3rds of maintenance
- 6 hourly U+E, with adjustment of fluid input accordingly
If symptomatic, then discuss with on call consultant about the need for 3% saline at 1-2 ml/kg/hr (0.5-1 mmol/kg/h) for two to three hours.
3. Cerebral salt wasting (CSW) -(Rare)
Following neurosurgery or in severe CNS trauma and in some cases of spontaneous intracerebral haemorrhage), polyuria and volume depletion may occur but accompanied by very high urine sodium losses (much greater than in SIADH) resulting in hyponatreamic dehydration and a urine:plasma osmolality ratio>1. This is treated as follows:
- Manage input of fluids as
Insensible losses (300ml/m2/day) (See BNF for Children –back pages to work out m2)- + estimated % dehydration replaced over 48 hr
- + previous hrs urine output
OR
Maintenance fluids - + estimated % dehydration replaced over 48 hrs
(The maintenance fluid should then be reviewed twice daily and adjusted according to fluid balance, weight and U+Es)
- However, sodium content of fluids should be increased significantly according to 6 hrly U+E. Sodium supplements can also be given orally.
4. Corticosteroid deficiency
Following neurosurgery, the patient may have inadequate ACTH and cortisol secretion, which may mask DI. The need for hydrocortisone replacement should be discussed with the on call consultant and may require a synacthen test to diagnose. Once hydrocortisone replacement is started, this may unmask or worsen DI requiring a change of dose of DDAVP. Therefore close monitoring is required as above during this time period. Most tumour patients will be on perioperative dexamethasone so in practice this is not usually an issue until after the acute perioperative phase when steroids are tapered.
|
Consider DDAVP administration if: Urine output 5ml/kg/hour for 2 consecutive hours AND Plasma sodium > 145mmol/L >AND Urinary Specific Gravity (SG) < 1.005 |
ACCURATE DIAGNOSIS OF SODIUM / WATER BALANCE IN NEUROSURGICAL PATIENTS DEPENDS ON MONITORING TRENDS
May need HOURLY assessments of
- Serum Na
- Urine volumes
- Urine Na
- Serum osmolality
- Fluid Balance
|
SERUM NA |
URINE VOL |
URINE NA |
SERUM OSM |
FLUID BAL |
|
|
Rising/ high |
High |
Low |
High |
Negative |
Diabetes |
|
Low |
Low |
Normal/High |
Low |
Positive |
SIADH |
|
Low |
High |
High |
Normal |
Negative |
Cerebral salt |
Last reviewed: 04 November 2019
Next review: 01 November 2022
Author(s): G Shaikh
Version: 4
Approved By: Paediatric Clinical Effectiveness & Risk Committee

