Hyperkalaemia, emergency management of, PICU
exp date isn't null, but text field is
Objectives
-
to standardise the emergency management of hyperkalaemia in children cared for by the PICU team at RHC
-
to provide an evidence base for the proposed guideline
-
to provide background educational information to allow practitioners to understand the principles of the therapy
Scope
-
Emergency management of hyperkalaemia
-
This guideline is intended for the management of hyperkalaemia in patients in paediatric critical care. Transfer to a high dependency or PICU environment should be considered depending on where the patient is located. According to the patient’s condition, it may be appropriate to initiate and complete management within the A+E Department, Oncology, Cardiology or Renal Wards. Renal & Oncology patients may have their own treatment algorithm.
-
Hyperkalaemic chronic renal patients should be discussed with both consultant Renal and consultant PICU staff as a matter of urgency. Oncology patients with new onset severe hyperkalaemia (e.g. Tumour Lysis Syndrome) should also be discussed with both the Consultant Oncologist and Consultant Intensivist.
NB: If you are reading this because your patient currently has hyperkalaemia, please INITIATE IMMEDIATE ECG MONITORING AND A 12 LEAD ECG, then follow the EMERGENCY TREATMENT ALGORITHM below.
The absence of ECG changes in cases of moderate or severe hyperkalaemia does NOT obviate the need for therapy; while the presence of ECG changes, even in mild hyperkalaemia, should initiate PROMPT AND AGGRESSIVE TREATMENT.
PICU FELLOW ON-CALL, DECT PHONE: 84725
PICU GENERAL CONSULTANT, DECT PHONE: 84719
Audience
- Critical care personnel managing a child who has hyperkalaemia in the Royal Hospital for Children, Glasgow. This guidance may also be referred to by NHS staff in other RHC departments or other health practitioners who are managing such a child in concert with RHC critical care colleagues.

Potassium is a mostly intracellular cation with approximately 98% of the total being within the intracellular fluid (ICF) compartment (mainly muscle) and only 2% in the extracellular fluid (ECF). This ratio of ECF:ICF potassium is a primary determinant of resting membrane potential in all electrically active cells and a small absolute change in extracellular potassium can alter the resting membrane potential of these cells. This can have dramatic effects on cell function including altering myocardial cell conduction velocity, repolarisation, preventing normal muscle contraction and nerve functioning. Therefore the body maintains tight control of extracellular potassium in a variety of ways including altering renal and gut excretion and the flux of potassium into or out of the intracellular compartment (mainly via the sodium potassium pump).
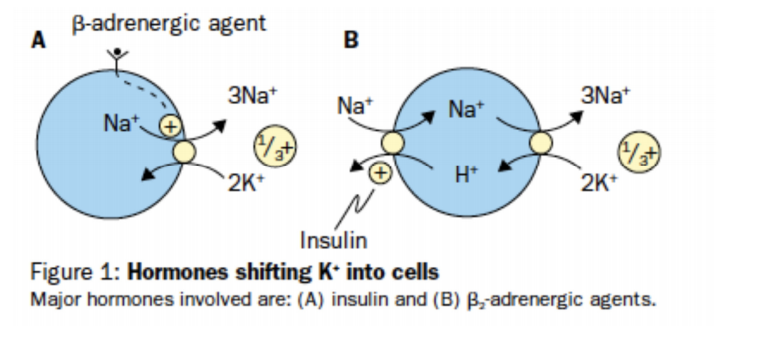
Potassium is retained in cells by a negative voltage, generated by the active transport of Na out of cells by Na/K-ATPase. Both insulin and catecholamines cause a potassium shift into cells. Insulin stimulates the NA/H exchanger, the intracellular Na is now available for exchange with Potassium via Na/K-ATPase, whilst catecholamines directly activate the Na/K-ATPase via adenlate cyclase activation, which in turn stimulates cAMP which is utilised by Na/K-ATPAse . The concentration gradients of Na and K produce an electrical potential across the myocyte leading to a resting membrane potential of -90mV.
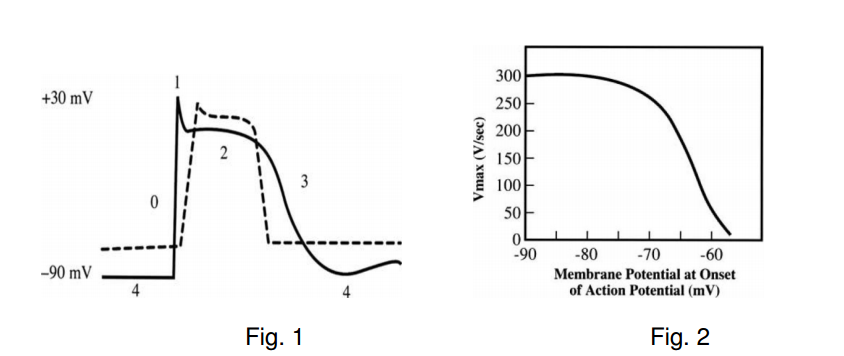
As ECF K content increases the concentration gradient across the myocyte decreases and the resting membrane potential is lowered (Fig.1) The rate of rise of phase 0 of the action potential (Vmax) is directly proportional to the value or the resting membrane potential at the onset of phase 0 (Fig.2). The decrease in Vmax slows conduction and prolongs membrane depolarisation. In addition the duration of the action potential is decreased. Initially hyperkalaemia increases excitability by shifting the resting membrane potential to a less negative value i.e. nearer to the threshold potential. Subsequently Vmax continues to decrease and myocyte depression occurs.
Hyperkalaemia is defined as a potassium level greater than the upper limit of normal for age. Normal serum (extracellular) potassium levels are age dependant in children, within the range 3.5 to 5 mEq/l (mmol/l), but can be as high as 6.mEq/L in premature infants [1]. There is no universally accepted definition for mild, moderate, or severe hyperkalaemia. [2] Some authors suggest mild to be 5.5 to 6 moderate 6.1 to 7, and severe hyperkalaemia to be greater than 7mEq/l (mmol/l) [3].
Hyperkalaemia is not uncommon with some reports suggesting up to 10% of hospitalised adult patients being affected [4], though in reality the incidence in the paediatric population is unknown. It can be a serious and potentially life threatening condition and may necessitate urgent intervention that often precedes complete investigation of the cause.
Unfortunately, serum potassium does not always correlate with severity of the clinical picture and other factors such as rapidity of rise, acid-base status and pre-morbid condition of the patient also need to be considered. Classic electrocardiogram changes associated with hyperkalaemia are well described in the literature [5]. However, it is important to appreciate that the relationship between serum potassium and ECG changes varies between individuals particularly in mild to moderate elevation of potassium. In some patients with severe hyperkalaemia, particularly of a more chronic nature, minimal ECG changes may be evident [5] [6]. As such ECG changes should not be taken in isolation to determine management and it is important to note that a normal ECG should not preclude need for treatment.
The most common cause of hyperkalaemia in paediatric practice is pseudohyperkalaemia or a spuriously elevated result. The clearest indicators of probable spurious hyperkalaemia are clinical context and renal function [7].
Therefore, if the patient has normal renal function and no risk factors for hyperkalaemia it is likely that the result is spurious.
(1) Pseudo-hyperkalaemia may be due to:
- Excessive tourniquet time, fist clenching
- Squeezing related to capillary blood letting
- Sampling proximal to a line taking potassium containing fluid
- Sampling a line with potassium containing fluid
- Sampling from a central line lumen distal to lumen containing potassium infusate
- Vigorous shaking of sample
- Contamination of sample by potassium EDTA from FBC bottle
- Injection of serum through narrow bore needle into sample bottle
- Delay in processing of sample
- Cold storage/transport of sample (big issue in primary care)
Some of these situations cause red cell haemolysis. This “in vitro” haemolysis causing potassium to leach out of red cells can usually be detected in the laboratory by a pinkish tinge to the serum.
True hyperkalaemia is due to a combination of increased intake, transcellular shift and decreased excretion of potassium.
(2) Renal dysfunction or renal tract obstruction:
This can be chronic and includes patients on various dialysis regimens, or acute in which case it may not be known at presentation that the patient has renal dysfunction.
(3) Conditions that cause cellular potassium leakage including:
- Marked leucocytosis/leukaemia
- Thrombocytosis (potassium released during clot formation-plasma levels normal serum levels raised)
- Hereditary and acquired red cell disorders causing in vivo haemolysis
- Tumour lysis syndrome
- Trauma, burns, surgery, crush injury, rhabdomyolysis
- Compartment syndrome
(4) Causes of increased potassium intake or reduced excretion including:
|
}commonest . . . . . .
|
(5) Causes of transcellular shift of potassium out of cells:
- Acidosis
- Some drugs e.g. β-blockers, suxamethonium* , propofol
- Digoxin toxicity
- Insulin deficiency
- Cessation of β-adrenergic agonists, followed by leaching of K+ out of cells
* Suxamethomium can cause a transient rise in serum potassium of 0.5- 1mmol/l for up to half an hour [8][9][10]
(6) Rare conditions such as:
- Hyperkalaemic familial periodic paralysis
- Disorders of adrenal insufficiency, hypoaldosteronism and Addison’s (inadequate aldosterone driven excretion of potassium)
- Malignant hyperthermia
Hyperkalaemia may be entirely asymptomatic and detected coincidentally during blood testing for another reason or the patient may have symptoms such as tiredness, nausea, vomiting, muscle weakness, palpitations or very rarely flaccid paralysis.
Even in the absence of symptoms a high potassium level particularly in conjunction with supportive ECG changes is a medical emergency. A totally asymptomatic patient can rapidly progress to develop a life threatening arrhythmia in this condition. It is therefore vital that the diagnosis is reached rapidly and that appropriate therapeutic measures are undertaken immediately.
As already mentioned in paediatric practice spuriously high potassium levels are a regular occurrence often due to difficulty obtaining blood samples. However these “possibly spurious” results must never simply be ignored, and should always be repeated.
The clinician should determine whether the patient has any signs of renal dysfunction or if any other features in the history or examination make him at risk of hyperkalaemia (e.g. drug history, known diabetic, patient receiving chemotherapy). Indicators of renal function including urea and creatinine will usually be available concurrent with the potassium result to provide some guidance.
In most cases of unexpected hyperkalaemia it is prudent to rapidly repeat the test whilst preparing treatment, though treatment should not be delayed awaiting the result, especially if there are ECG changes supportive of elevated potassium [11]. In many acute paediatric assessment areas there is now the ability to measure a potassium level on a blood gas analyser which will take a matter of minutes. Whilst a specimen should also be sent to the laboratory for confirmation, therapy if needed should not be withheld awaiting the formal laboratory result.
Whilst confirming the diagnosis and during therapy, the patient should have continuous ECG monitoring, to detect any of the classic changes associated with hyperkalaemia. Whilst there is a recognised progression of ECG changes from mild through to severe hyperkalaemia, there is a poor correlation between ECG changes and serum potassium concentration, and the progression of rhythm abnormalities is unpredictable in any individual. ECG changes are dependent on both the absolute value and the rate of increase of potassium levels. Some patients with severe hyperkalaemia may have no identifiable ECG changes and others may have changes at surprisingly low potassium levels. The absence of ECG changes in cases of moderate or severe hyperkalaemia does not obviate the need for therapy. The presence of ECG changes, even in mild hyperkalaemia, should encourage PROMPT AND AGGRESSIVE treatment.
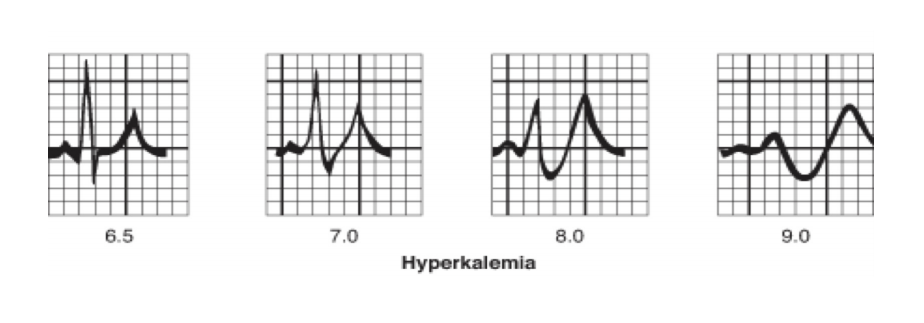
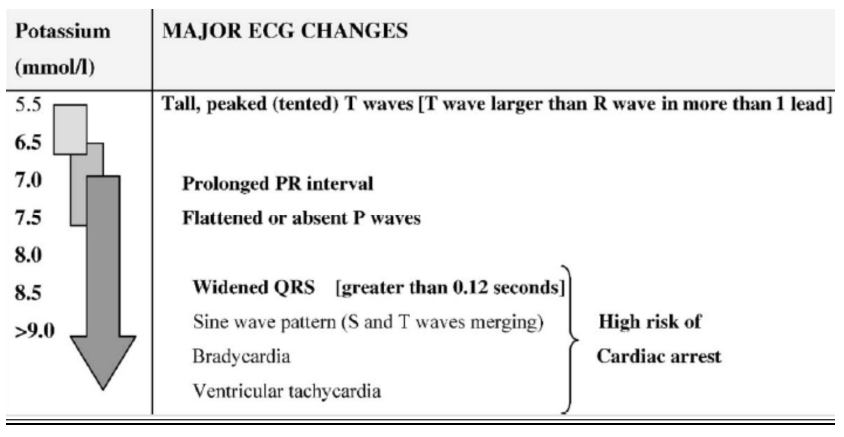
Early ECG changes in mild hyperkalaemia (>5.5mEq/l) include peaking or tenting of the T-wave. Thereafter as levels exceed 6.5mEq/l there is reduction in conduction velocity resulting in prolonged PR interval and progressive widening of the QRS complex and nodal and ventricular arrhythmias may occur. Ultimately the p-waves disappear and the QRS complexes combine with the T-wave to form a sine-wave appearance with ventricular fibrillation or asystole ensuing.
Regular monitoring of serum potassium, electrolytes, glucose and blood gas are useful especially as hyperkalaemia is frequently associated with acidosis and hyperglycaemia. In some cases it may be useful to perform:
- Full blood count with differential and film to look for haemolysis, leucocytosis and thrombocytosis
- Creatinine kinase in cases of possible tissue injury, trauma, burns
- Urinary potassium excretion
- Bilirubin, reticulocytes and haptoglobin in suspected haemolysis
- Renal ultrasound if suggestion of renal dysfunction or obstruction
These investigations depend upon the individual clinical situation and must never delay acute treatment once it is deemed necessary.
Airway, Breathing, Circulation.
Repeat serum potassium should be ordered urgently, especially if hyperkalaemia is an unexpected or isolated finding and there are no ECG signs of hyperkalaemia, to exclude pseudohyperkalaemia. This can be run on the gas machine and should not preclude starting preparation of definitive therapy.
Hypoxia potentiates the risk of cardiac dysrhythmia, so all patients (unless there is an associated cardiac lesion requiring low FiO2) should be administered oxygen.
The response of an individual patient to any of the following proposed therapies is unpredictable, so frequent repeated monitoring of potassium is mandatory.
ECG monitoring should be continuous once hyperkalaemia is suspected and treatment is commenced. Emergency treatment is required in any patient with ECG changes, is symptomatic or who has a true potassium level above 6.5mmol/l regardless of ECG appearance. Between 6 and 6.5mmol/l calcium may be withheld at the discretion of senior medical staff but other therapy should be commenced. Even lower levels of hyperkalaemia may require treatment particularly in the setting of a rapid or acute rise when it is less well tolerated. Arrhythmia control is difficult without lowering the serum potassium level.
Ideally calcium and glucose 50% should be administered centrally. If central access is unavailable then it may be appropriate to give larger volumes of a more dilute concentration. Preparation time and risk of injuries secondary to extravasation must be weighed against the risk of cardiac instability.
Bradycardia secondary to hyperkalaemia presents a conundrum and mandates the PICU Consultant being informed. It is unlikely to resolve without correcting the hyperkalaemia but therapy with calcium salts may exacerbate the bradycardia, or produce AV block and asystole. The effect of atropine and phenylepherine is blunted. Pacing may prove ineffective because of increased stimulation thresholds secondary to hyperkalaemia. Standby ECMO may have to be considered.
There are four components to the acute management of hyperkalaemia:
- PROTECT - Antagonize the membrane toxic effects of potassium
- REDISTRIBUTE - Promote rapid cellular uptake of potassium
- ELIMINATE - Remove potassium directly from the body
- DISCONTINUE - Remove exogenous potassium sources (drugs, fluids)
Calcium salts antagonise the effects of hyperkalaemia on the cardiac membrane. They increase the threshold potential, restoring the gap between the resting membrane potential and the threshold potential. In addition it restores Vmax at higher resting membrane potentials, normalising the rate of myocyte depolarisation, and normalises impulse propagation in the SA and AV nodes. There is no effect on serum potassium levels and other therapies that lower serum potassium levels should be administered concomitantly.
Either 10% calcium gluconate or 10% calcium chloride can be used depending on availability. Calcium chloride has 3 times more elemental calcium than an equal volume of calcium gluconate, so if volume administration/tolerance is a problem then theoretically calcium chloride may be preferred. Calcium chloride is more likely to cause extravasation injury.
The benefits of this treatment are usually evident with improvement in ECG changes within a few minutes and last 30-60 minutes. Doses as follows:
- IV Calcium Gluconate 10%, 0.5 -1ml/kg (0.11-0.22mmol/kg) [max 4.4mmol]
- IV Calcium Chloride 10%, 0.2ml/kg (0.14mmol/kg) [max 1.4mmol]
Administer over 5minutes and repeat as necessary after 5 minutes, if ECG fails to improve or deteriorates. The initial dose can be given over 1-3 minutes if life threatening arrhythmias or sine wave ECG changes are present. (Stop infusion if bradycardia develops) Administration should be over 30 minutes if the patient is on digoxin, to avoid hypercalcaemia that may potentiate the myocardial toxicity of digoxin.
These interventions buy time for more definitive therapy but they do not remove potassium from the body. Beta-blockers and digoxin may reduce the effectiveness of insulin-glucose and beta-2 agonists.
a) Insulin
Insulin stimulates the Na-K ATP pump to promote intracellular potassium uptake. It is thought to recruit intracellular pump components into the cellular membrane and increase availability of intracellular Na by its effect on the Na/H exchanger. It is indicated in every case of hyperkalaemia that requires treatment [11]. Insulin is co administered with glucose to prevent hypoglycaemia. Blood glucose should be checked at the end of the infusion and then every 15 minutes for an hour after administration as delayed hypoglycaemia is common.
The onset of action is around 15 minutes and the effect lasts for over 1 hour. Potassium levels have been shown to fall by up to 0.5mmol/l in 20minutes and 1mmol/l by an hour, so it is an effective temporising manoeuvre [11] [14]. Dosing can be repeated after 30 minutes. The effect lasts up to 4 hours. Dose as follows:
- 2ml/kg 50% dextrose (1g/kg) and 0.1units/kg of fast acting Insulin over 5-10 minutes (mixed in SAME SYRINGE) [13].
Theoretically one could give a dextrose load alone as this will increase the child’s own insulin production and promote intracellular uptake of potassium, however the potassium lowering effect is likely to be greater if glucose and insulin are used in combination. Also the endogenous insulin response may be inadequate and the resultant hypertonic state may exacerbate hyperkalaemia due to solvent drag with efflux of water from the intracellular to extracellular compartment.
Glucose may not need to be given if the serum glucose is > 16mmol/dl, but this should be discussed with the consultant before giving insulin alone.
b) β-adrenergic agonist.
Several paediatric studies, although small, advocate the use of salbutamol as a safe and effective measure to temporarily reduce serum potassium levels [15][16][17]. Salbutamol, (IV or nebulised), indirectly, via cyclic-AMP, stimulates the cell membrane Na-K ATP pump in hepatic and muscle cells to promote cellular potassium uptake. In addition it increases endogenous insulin secretion. This is secondary to hepatic gluconeogenesis derived hyperglycaemia, and is not a dominant mechanism for reducing potassium levels. Salbutamol is equally effective in diabetic and non diabetic patients and in diabetic patients where c-peptide levels are not elevated. [18]
There is controversy regarding use of salbutamol as some studies suggest it to be arrythmogenic at high dose. Salbutamol is also unlikely to be effective if the patient is on beta blockade for renal failure associated hypertension. There is also a significant proportion of patients (up to 20-40%) who are non-responders to this treatment with no or minimal reduction in serum potassium. The mechanism is unclear and unpredictable. For this reason it is recommended that salbutamol not be used alone in the treatment of severe hyperkalaemia. The dose is as follows:
- 4micrograms/kg IV salbutamol over 5 minutes repeated as required OR:
- 2.5mg (<5 years) or 5mg (>5years) nebulised salbutamol
Both of these approaches have been proven to be effective in reducing potassium levels by up to 1.4mmol/l if intravenous route used and up to 1mmol/l if nebulised. The onset of action is around 30 minutes, and the effect lasts for 2-3 hours. [15][16][17]
A few studies suggest a synergistic effect in using salbutamol combined with insulin/glucose with greater reduction in potassium level than either used alone. [14][18]. They are equally efficacious in lowering plasma potassium. The effect of salbutamol is delayed slightly relative to insulin but this is likely to be secondary to the mode of administration ie slow nebulisation as opposed to IV bolus. In addition salbutamol may offset the hypoglycaemic effect of insulin. This is consistent with β2-agonistic activity on the liver where stimulation of gluconeogenesis and glycogenolysis occurs. [18]
A Cochrane review suggests that Dextrose/Insulin and salbutamol are the first line therapies most supported by evidence, and that a combination of the two therapies may be more effective than either alone. [20] There is no statistically significant difference between the effectiveness of intravenous, nebulised or inhaled salbutamol in the limited number of studies to date. It is likely to be easier and quicker to nebulise a patient in the short term, though in the emergency situation in a non ventilated patient, delivery may be erratic. Under such circumstances intravenous follow up can be considered.
NB: Salbutamol can cause a transient release of potasssium from liver causing a small increase in potassium.
c) Sodium Bicarbonate
The use of sodium bicarbonate is no longer advocated for hyperkalaemia unless in the presence of significant metabolic acidosis. It has little effect in lowering serum potassium levels within 60 minutes when studied in adult populations with chronic renal failure [21][22]. It may offer slight reduction in potassium levels over a period of several hours particularly on the background of proven metabolic acidosis with pH < 7.2 [23]. Acidosis may inhibit the physiologic response to catecholamines and insulin, and using bicarbonate in conjunction with insulin or salbutamol may be of some benefit.
There is a risk of hypocalcaemia with sodium bicarbonate because the alkalosis it induces will decrease the plasma level of ionised calcium - this may be poorly tolerated and potentiate arrhythmias in the context of hyperkalaemia. It should not be administered at the same time via the same line as calcium or calcium bicarbonate may precipitate.
It is unclear whether any reduction in potassium is due to an intracellular shift of potassium with alkalinisation or if it is related to the large hypertonic load from large quantities of sodium in the preparation causing fluid shifts. Regardless, the significant side effects such as volume and sodium overload, particularly in patients with renal dysfunction, along with the knowledge that it is poorly effective, probably preclude its use in most situations. The evidence is, however, sufficiently inconclusive in cases of co-existing metabolic acidosis that some centres continue to use bicarbonate to manage hyperkalaemia in combination with other therapy. [24]. Dose as follows:
- 1mmol/kg over 10 minutes (repeat in 10 minutes as required; monitor blood gases to maintain pH <7.55)
a) Cation exchange resins e.g. calcium resonium and Resonium A
The degree of benefit of these treatments in hyperkalaemia remains controversial.
Cation exchange resins remove potassium directly from the body through the gut mucosa of the large bowel and ileum. As the resin passes through the colon, it comes into contact with fluids that contain increasing amounts of potassium. In the caecum the concentration of Na+ and K+ are similar to those in the small intestine. In the stool water of the sigmoid colon there may be 6-38 mmol/L sodium and 14-44 mmol/L potassium [25]. The result is that luminal potassium is taken up in increasing amounts in exchange for calcium or sodium ions contained in the resin. This creates a concentration gradient across the bowel wall, resulting in a gradual fall in serum potassium. The length of time the resin remains in the body is a decisive factor in its effectiveness. For this reason oral administration is more effective than the use of enemas which should, if possible, be retained for 9 hours. This limits the utility of cation exchange resins in acute hyperkalaemia.
The efficiency of potassium exchange is unpredictably variable. The resin is not selective for potassium. In vitro, one gram of resin theoretically exchanges 1mmol Na+ or 0.5mmol Ca2+ for 1mmol of K+. In vivo, each gram of the resin only binds about 0.65 mmol of potassium [19] and the effect is highly variable and unpredictable.
The resin-bound potassium is then held in the intestinal lumen and excreted in the faeces. The onset of action is slow, in the order of 1-2 hours, with peak of 4-6 hours so the temporising steps in points 3.1 & 3.2 (above) must be undertaken in the first instance.
As mentioned previously, the resins can be administered rectally or orally. The preferred route is oral (except in neonates). If administered rectally the resin should be mixed with methylcellulose and retained for at least 60 minutes. Recommended doses are: 250mg/kg (max 15g) 3-4 times per day orally or rectally, repeated every 6-8 hours if required for children over 1 month of age. [13] The resin must not be combined with fruit juices as they contain high levels of potassium. If the rectal route is used the colon must be irrigated 6-12 hourly to prevent faecal impaction. Side effects include rectal mucosal irritation and ulceration/necrosis, faecal impaction, constipation and rarely bowel perforation. These are of particular concern if sorbitol is coadministered as a laxative [26, 27]. Hypercalcaemia may also occur with Calcium Resonium, especially in patients on dialysis.
The data regarding the effectiveness of exchange resins is conflicting [19, 20, 28, 29]. It is possible to excrete up to 12 mmol/l potassium via the gastro-intestinal tract in healthy adults however this is limited by stool volume. This is especially pertinent in a paediatric population who obviously have much lower stool volumes. Some authors also suggest that resins do not contribute to faecal potassium excretion above the effect of laxatives alone [30].
In spite of these reservations the GAIN (Guidelines and Audit Implementation Network) in Northern Ireland still advocate the use of exchange resins to remove potassium from the gut though accept that other measures must be used in the first instance due to delayed onset of action [4]
b) Loop diuretic e.g. Furosemide
By blocking the NKCC2 co-transporter in the thick ascending limb of the Loop of Henle, these drugs increase urine flow and delivery of sodium to distal tubular/collecting duct potassium-excreting sites where more potassium is lost in exchange for sodium. This is likely to be less effective in patients with renal dysfunction who may have limited response to diuretics.
c) Haemodialysis or haemofiltration
This is the definitive method of removal of potassium from the body. It is used in cases of severe hyperkalaemia or when other treatments have failed to provide a sustained reduction in potassium. Rapid haemodialysis will remove potassium faster that Continuous Veno-venous Haemofiltration (CVVH), but in a critically unwell patient, CVVH may result in less haemodynamic instability due to fluid shifts.
Serum potassium can be lowered by 1-1.5mmol/l for every hour of dialysis although some rebound is expected on completion of dialysis. Rapid liaison with tertiary paediatric nephrologists or intensive care specialists capable of providing these treatment modalities is necessary when potassium is very high, other treatments appear to be failing, or if ongoing tissue damage and continued release of intracellular potassium is expected.
Drug treatments that cause intracellular shifts of potassium will decrease the efficacy of potassium clearance by dialysis because they decrease the concentration gradient between plasma and the dialysate. It may be necessary to re-dialyse this patient group as the effects of β-agonists’ wear off and potassium returns to the extracellular compartment. [31]
Potassium free replacement fluid or dialysate will maximise potassium clearance.
Review diet and reduce potassium intake - in normal individuals the kidneys can adapt to excrete excess potassium intake however this is not the case if there is kidney dysfunction or the patient is taking medications that alter potassium excretion such as potassium sparing diuretics. Notably “Lo salt” replaces sodium salt with potassium salt and may be an occult contributory factor.
Review of medications and fluid/nutrition prescriptions - potassium sparing diuretics, ACE inhibitors, heparin, trimethoprim and other potassium containing antibiotics, Fybogel and Movicol all act by various mechanisms to increase extracellular potassium levels [13, 32].
Renal Replacement Therapies – haemodialysis (HD) is the definitive treatment of hyperkalaemia. Particularly useful in cases of acute or chronic renal failure or when other treatments have failed and the potassium level remains high. If a potassium free diasylate is used, serum potassium may decrease as much as 1.2 - 1.5mmol/l per hour, however there will be rebound in potassium levels on completion of dialysis due to equilibration from the intracellular compartment. Peritoneal dialysis is poorly effective at lowering serum potassium levels in comparison. As mentioned above, CVVH with a potassium-free replacement fluid, although slower in action than HD, can be useful in the cardiovascularly unstable PICU patient.
Longer term treatment must be individual patient based, dependent upon the cause of the hyperkalaemia. It may simply be a case of dietary restriction or alteration to drug regime though in other cases, such as hypoaldosteronism, mineralocorticoid replacement therapy would be necessary or haemodialysis may be required for renal dysfunction.
- Shaffer SG, Kilbride HW, Hayen LK, Meade VM, Warady BA. Hyperkalemia in Very Low Birth Weight Infants. J Pediatr, 1992;121:275-9.
- Brenner BM. Brenner & Rector's The Kidney, 7th ed. 2004. Philadelphia: Saunders.
- Mandal AK. Hypokalemia and hyperkalemia. Med Clin North Am 1997;81:611–39
- Guidelines for the Treatment of Hyperkalaemia in Adults GAIN (Guidelines and Audit Implementation Network- Northern Ireland) August 2014
- Webster A, Brady W, Morris F. Recognising Signs of Danger: ECG Changes Resulting From an Abnormal Serum Potassium Concentration. Emerg Med J 2002;19:74–77
- Martinez-Vea A, Bardaji A, Garcia C, Oliver J A. Severe Hyperkalemia With Minimal Electrocardiographic Manifestations. Journal of Electrocardiology1999 32 1 45-49
- Stuart W, Smellie A. Spurious Hyperkalaemia. BMJ 2007;334:693-5
- Day S. Plasma Potassium Changes Following Suxamethonium And Suxethonium In Normal Patients And In Patients In Renal Failure Br.J. Anaesth. 1976;48: 1011-15
- List W F. Serum Potassium Changes During Induction Of Anaesthesia. Brit. J. Anaesth. 1967;39: 480-484
- Weintraub H D, Heisterkamp D V, Cooperman L H. Changes In Plasma Potassium Concentration After Depolarizing Blockers In Anaesthetized Man. Brit. J. Anaesth. 1969;41:1048-105
- Ahee P, Crowe AV. The Management Of Hyperkalaemia In The Emergency Department. J Accid Emerg Med 2000;17:188–191
- Noyan A, Anarat A, Pirti M, et al. Treatment Of Hyperkalemia In Children With Intravenous Salbutamol. Acta Paediatr Jpn 1995;37:355–7
- Martin J (ed) BNF for Children 2010. London: Pharmaceutical Press 2010
- Lens XM, Montoliu J, Cases A, et al. Treatment Of Hyperkalemia In Renal Failure: Salbutamol V Insulin. Nephrol Dial Transplant 1989;4:228– 32
- Murdoch IA, Dos Anjos R, Haycock GB. Treatment Of Hyperkalemia With Intravenous Salbutamol. Arch Dis Child. 1991;66:527-8
- Kemper MJ, Harps E, Hellwege HH, et al. Effective Treatment Of Acute Hyperkalaemia In Childhood By Short term Infusion Of Salbutamol. Eur J Pediatr 1996;155:495–7
- McClure RJ, Prasad VK, Brocklebank JT. Treatment Of Hyperkalaemia Using Intravenous And Nebulised Salbutamol. Arch Dis Child 1994;70:126–8
- Allon M, Copkney C. Albuterol And Insulin For Treatment Of Hyperkalemia In Hemodialysis Patients. Kidney Int 1990;38:869–72
- Weisberg, LS. Management of Severe Hyperkalemia, Crit Care Med 2008; 36: 3246 –3251
- Mahoney BA, Smith WAD, Lo D, Tsoi K, Tonelli M, Clase C. Emergency Interventions For Hyperkalaemia (Review). Cochrane Collaboration July 2009
- Allon M, Shanklin N. Effect Of Bicarbonate Administration On Plasma Potassium In Dialysis Patients: Interactions With Insulin And Albuterol. Am J Kidney Dis 1996;28:508–14
- Blumberg A, Weidmann P, Shaw S, et al. Effect Of Various Therapeutic Approaches On Plasma Potassium And Major Regulating Factors In Terminal Renal Failure. Am J Med 1988;85:507–12
- Allon M. Treatment And Prevention Of Hyperkalemia In Endstage Renal Disease. Kidney Int 1993;43:1197–209
- Kamel K S, Wei C. Controversial Issues In The Treatment Of Hyperkalaemia. Nephrol Dial Transplant 2003; 18: 2218–2221
- Sanofi-Aventis Canada Inc. Prescribing Information for RESONIUM CALCIUM® Cation-Exchange Resin (calcium polystyrene sulfonate) 2905 Place Louis R.-Renaud, Laval, Quebec H7V 0A3, Canada Date of Revision: July 9, 2014. Submission Control No.: 172950.
- Lillemoe KD, Romolo JL, Hamilton SR, et al. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: Clinical and experimental support for the hypothesis. Surgery 1987; 101:267–272
- Rogers FB, Li SC: Acute colonic necrosis associated with sodium polystyrene sulfonate (Kayexalate) enemas in a critically ill patient: Case report and review of the literature. J Trauma 2001; 51:395–397
- Watson M, Abbott KC, Yuan CM Damned If You Do, Damned If You Don't: Potassium Binding Resins in Hyperkalemia. Clin J Amer Soc Nephrol.2010; 5(10): 1723-1726
- Elliott MJ, Ronksley PE, Clase CM, Ahmed SB Hemmelgarn BR. Management of patients with acute hyperkalemia. CMAJ 2010; 182(15): 1631-1635
- Gruy-Kapral C, Emmett M, Santa Ana CA. Effect Of Single Dose Resin-Cathartic Therapy On Serum Potassium Concentration In Patients With End-Stage Renal Disease. J Am Soc Nephrol 1998; 9: 1924–1930
- Allon M, Shanklin N. Effect Of Albuterol Treatment On Subsequent Dialytic Potassium Removal. Am J Kidney Dis 1995;26:607-1
- Raebel, MA. Hyperkalemia Associated with Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers. Cardiovascular Therapeutics 2012; 30: e156–e166
Last reviewed: 15 December 2016
Next review: 10 December 2020
Author(s): Colin Begg
Reviewer Name(s): Paediatric Clinical Effectiveness & Risk Committee
Document Id: 387

