Less Invasive Surfactant Administration (LISA), neonatal guideline
exp date isn't null, but text field is
Audience
This Guideline is relevant to all neonatal units managing preterm infants in the West of Scotland. Users should also refer to the West of Scotland guidelines for respiratory support of the preterm infant and for Intubation and premedication. They should also refer to the Neonatal pharmacy monographs for all of the medications used.
Preterm infants commonly develop respiratory distress syndrome (RDS) requiring some form of respiratory support and potentially surfactant administration1. Treatment with surfactant has been shown to reduce the risk of death and bronchopulmonary dysplasia (BPD) in preterm infants; however the standard approach to administering surfactant involves using an endo-tracheal tube and a period of mechanical ventilation (MV)2. It is well known that MV causes damage to the fragile preterm lungs so many lung protective strategies for respiratory management and ventilation have been developed.
As per our current guideline on the respiratory management of preterm infants the preferred initial management is using primary CPAP with the use of rescue surfactant for infants with an increasing oxygen requirement. To reduce the need for MV but still deliver surfactant several less invasive techniques have been developed. One of these techniques is called LISA and is the process of delivering surfactant directly into the lungs via a fine bore catheter inserted into the trachea3. In a recent review by Wu et al4, LISA technique was associated with a significant reduction in the risk of mechanical ventilation within 72hours and bronchopulmonary dysplasia at 36 weeks compared to other strategies of surfactant administration (p<0.00001). These findings have been also been seen in a meta-analysis by Isayama et al in 20151 and most recently by Aldana-Aguirre et al who found a lesser need for MV and a reduction in the composite outcome of death or BPD at 36 weeks compared to INSURE (p 0.01)2.
Infants who are suitable to be considered for LISA are those being managed with primary CPAP or High Flow with evidence of increasing respiratory distress and with a rising oxygen requirement. Recent evidence suggests that the threshold of fiO2 of 30% should be used for all infants <32 weeks gestation to reduce complications in untreated RDS6. In infants <32 weeks gestation it can be assumed that their oxygen requirement is most likely surfactant deficiency and should be given surfactant via LISA without waiting for a chest x-ray to confirm the diagnosis. In infants >32 weeks gestation with an oxygen requirement of 30% or more, it may be beneficial to undertake a chest x-ray to rule out any other causes for the respiratory distress, prior to giving surfactant. If RDS is diagnosed, surfactant should be given promptly. In units where this technique is still relatively new, it is advisable that it is performed under the controlled environment within neonatal intensive care, as teams become more confident there may be scope for using this technique in the delivery room.
- Perform LISAin infants <32 weeks gestation with any persistent or rising fiO2 requirement, following stabilisation on non-invasive respiratory support. The aim is to treat with surfactant before the fiO2 requirement reaches 30%, at any time in the first 72 hours. Or, as soon as possible if the FiO2 is already >30% following stabilisation.
- Perform LISAin infants >32 weeks gestation with a persistent or rising fiO2 requirement >30% following stabilisation on non-invasive respiratory support, if x-ray confirms the diagnosis of surfactant deficiency. Treatment may be considered at any time in the first 72 hours. At consultant discretion, LISA may be performed, without awaiting radiological diagnosis, in an infant with a higher initial oxygen requirement (fiO2 > 40%)
N.B. Consider pneumothorax in any infant who experiences a rapid rise in FiO2
Absolute Contraindications
- Imminent need for intubation as judged clinically by the attending senior clinician
- Maxillo-facial, tracheal or known pulmonary malformations
- Alternative cause for respiratory distress eg. Congenital pneumonia
- No experienced personnel available to perform LISA
Relative Contraindications
- Severe RDS with high oxygen requirements, severe respiratory acidosis and/or widespread atelectasis on chest x-ray.
- A suggested threshold for intubation being preferable over LISA is fiO2 > 50% in < 32 weeks gestation and > 60% in those > 32 weeks gestation.
- Consultant discretion
- Infants < 26 weeks gestation in a unit just starting to use LISA
- Pneumothorax requiring drainage
- Prominent apnoea despite adequate caffeine citrate administration
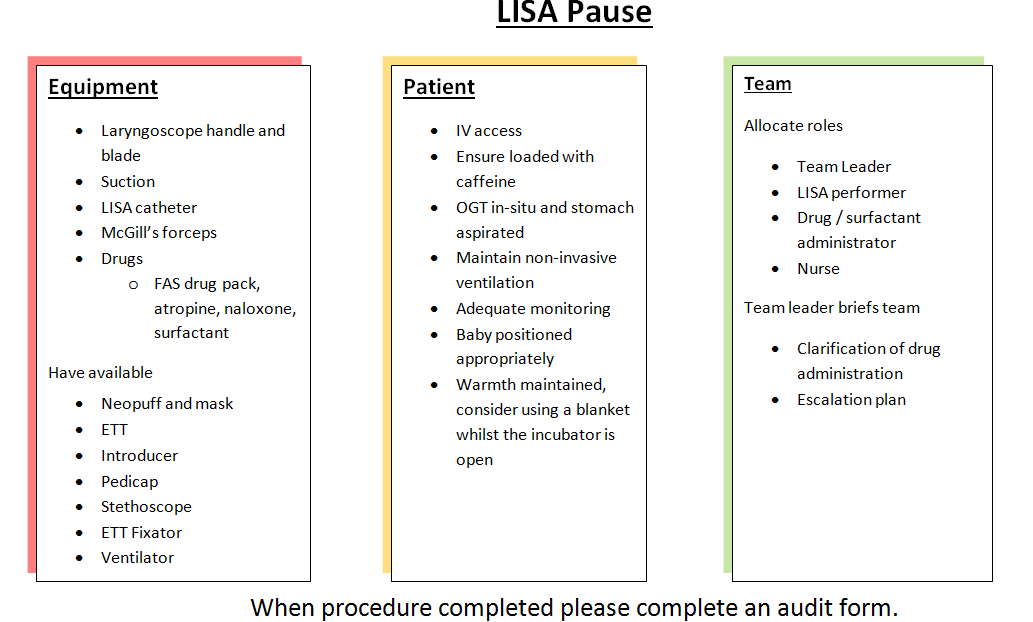
Preparation for LISA is very similar to that for elective intubation. The infant should be positioned as if for intubation and swaddled for comfort. It is important that the baby has continuous monitoring of heart rate and oxygen saturations. They should have an orogastric tube in-situ and the stomach should be aspirated. The baby should have intravenous access which has been confirmed to be working. It is important to maintain the baby’s temperature throughout the procedure. Ensure that the infant has been commenced on appropriate dose of caffeine citrate and dose given at least 30minutes prior to procedure.
A neoouff or self inflating bag and appropriate sized mask should be available as well as appropriately sized endotracheal tubes should the procedure have to be abandoned due to persistent hypoxaemia, bradycardia or apnoea. Surfactant should be drawn into a 5ml syringe to be delivered through the cords via a LISA catheter. The video laryngoscope can be used to insert the LISA catheter. This gives opportunity for correct placement of the catheter through the vocal cords to be confirmed by an assistant. However should the operator not be comfortable or experienced with the use of the video laryngoscope and are confident with direct laryngoscopy then a standard laryngoscope can be used. CPAP or High Flow should be maintained throughout the procedure. In some instances the CPAP prongs may obscure the view for laryngoscopy so for the duration of the procedure the infant may need to be managed on High Flow.
- Infant loaded with caffeine citrate prior to LISA
- Continuous heart rate and saturation monitoring
- OGT with stomach aspirated
- IV access
- Maintain temperature
- Neopuff/self inflating bag and mask
- ETT, fixator and Pedicap
- LISA catheter
- Surfactant 200mg/kg to nearest vial drawn up into a 5ml syringe
- Video or standard laryngoscope with appropriate sized blade
No RCT’s have looked at the use of premedication for LISA but expert opinion suggests that premedication may be considered. It is known that laryngoscopy causes adverse physiological responses including systemic, pulmonary and intracranial hypertension and bradycardia, but with the use of a premed these effects as well as pain and discomfort can be reduced. A retrospective review by Dekker et al showed there was improved comfort during LISA when sedation was given (p = 0.002), however there was a slight trend to more ventilation when it was used7. Some practices are to give ‘awake sedation’ with naloxone available should there be opiate induced apnoea or shallow breathing8. Atropine should be available to be given in instances of prolonged bradycardia, it should be noted that atropine can cause urinary retention and this should be observed for following administration.
For some infants non-pharmacological methods may be all that is required to perform LISA. In the less mature infant the use of sucrose and swaddling seems to be tolerated very well and reduces the risk of apnoea associated with fentanyl administration. In some of these infants consideration should be given to using atropine routinely. Fentanyl should be considered in the more mature and vigorous infants.
- Sucrose
- Fentanyl 1microgram/kg IV given slowly
- Surfactant 200mg/kg to nearest vial via LISA catheter
- Have available IV atropine 15 micrograms/kg, naloxone 200micrograms, further dose of fentanyl 1microgram/kg.
- A top up dose of fentanyl to take up to 5microgram/kg and 2mg/kg of suxamethonium should also be available in case the infant needs to intubated and ventilated
LISA should be performed by or supervised by staff members who have been trained in how to carry out the procedure and who are competent at intubation. The video laryngoscope, where the operator is comfortable with its use, may be used for LISA procedures to assist in accurate positioning of the LISA catheter. Following administration of the fentanyl direct laryngoscopy should be performed and the LISA catheter inserted through the vocal cords to the desired length, 1.5cm for infants < 27 weeks gestation and 2cm for infants >27 weeks gestation. Occasionally McGills forceps may be required to place catheter through the cords. Once the catheter is seen passing through the cords the laryngoscope should be withdrawn while ensuring the catheter remains in-situ, taking note of the marking on the catheter which is at the lips. The mouth is then held shut to maintain CPAP. The surfactant is slowly injected into the catheter over 2-3 minutes. If there is prolonged hypoxia or bradycardia the rate of surfactant administration should be reduced. FiO2 should be titrated to keep oxygen saturation between 89 and 95%. If there is persistent bradycardia atropine should be administered. If the baby becomes apnoeic or has persistently shallow breathing despite gentle stimulation, thought to be secondary to the sedation a dose of naloxone should be given. If despite this or at anytime the baby has prolonged bradycardia, hypoxia or is apnoeic the procedure should be stopped and the baby given positive pressure ventilation plus or minus intubation.
- Appropriately trained personnel carrying out procedure
- Use video laryngoscope if comfortable with use
- LISA catheter inserted through cords to desired length
- Note length at lips to ensure catheter remains in same place throughout procedure
- Remove laryngoscope holding catheter in place
- Hold mouth closed
- Inject surfactant slowly over 2-3 minutes
- Titrate oxygen as required
- Atropine if persistent bradycardia
- Naloxone if apnoeic or shallow breathing despite stimulation
- Intubate if prolonged apnoea, bradycardia or hypoxia
- Aspirate OGT following procedure, looking for surfactant reflux into stomach
Following administration of surfactant the baby should be nursed prone on nasal CPAP or Duopap in an incubator. If the procedure has been successful there should be a marked reduction in oxygen requirement within hours of the procedure. If the baby develops an increasing oxygen requirement, a pneumothorax should be excluded. A further dose of surfactant should be considered. This could be administered by LISA if they do not meet any of the contra-indications, however if they have marked work of breathing, a persistently high oxygen requirement, a respiratory acidosis or severe RDS on chest x-ray they should be intubated and ventilated prior to the repeat dose of surfactant.
This is a new procedure being carried out in the unit and it is important to record how the babies who we perform this on tolerate the procedure and manage post procedure. Please complete an audit data collection form following completion of the procedure.
- Isayam T, Chai-Adisaksopha C, McDonald SD. Nononvasive ventilation with vs without early surfactant to prevent chronic lung disease in preterm infants: a systematic review and meta-analysis. JAMA Pediatrics 2015;169:731
- Aldana-Aguiree JC, Pinto M, Featherstone RM et al. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Childhood Fetal Neonatal Ed 2017;102:F17-F23
- Dargaville PA, Aiyappan A, Cornelius A et al. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed 2011;96:F243-F248
- Wu W, Yan S, Fengaxia L et al. Surfactant administration via a Thin endotracheal catheter during spontaneous breathing in preterm infants. Pediatr Pulmonol. 2017;52:844-854
- Sweet DG, Carnielli V, Griersen G et al. European consensus guidelines on the management of respiratory distress syndrome – 2016 update. Neonatology 2017;111:107-25
- Dargaville PA, Aiyappan A, De Paoli AG et al. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology 2013;104:8-14
- Dekker J, Lopriore E, Rijken Met al. Sedation during minimal invasive surfactant therapy in preterm infants. Neonatology 2016;109:308-313
- Reynolds P, Lillitos P. Implementing and improving less invasive surfactant administration (LISA). Infant 2017; 13(5), 193-195
Last reviewed: 15 November 2021
Next review: 01 November 2024
Author(s): Dr Carolyn Abernethy – Neonatal Consultant PRM
Co-Author(s): Other Professionals Consulted: Dr Joyce O’Shea – Neonatal Consultant RHC; Acknowledgement for work on previous iterations: Dr Nikolaus Kau – Neonatal Consultant Aberdeen
Approved By: MCN for Neonatology West of Scotland
Document Id: 965

