Humidified High Flow Nasal Cannulae (HHFNC)
Objectives
Humidified High Flow Nasal Cannulae (HHFNC) Delivered by - Precision Flow (Vapotherm), Optiflow (Fisher & Paykel) or Fabian (Inspiration Medical)
This guideline is applicable to all medical and nursing staff caring for babies in neonatal intensive care units in West of Scotland neonatal services. All staff using these devices must ensure they have received instruction in the safe use of these devices.
Humidified High-flow nasal cannulae (HHFNC) are small, tapered cannulae that are used to deliver heated, humidified high-flow air and blended oxygen. HFNC may deliver positive end expiratory pressure. They are usually less than 1cm long and should occlude less than 50% of the area of nares 1. Flow rates used are >1L/min.
The use of HHFNC in the UK and around the world in neonatal units is high 2,3. It is common in both term and preterm infants 3,4..
HHFNC are being used as an alternative to CPAP, weaning from CPAP, for apnoea of prematurity and post extubation 2,5,7. Reported advantages of HHFNC over CPAP are: less trauma to the nares, simple application, easier to care for and access the baby, including for skin-to-skin and feeding 1,2,3,6.
Reported benefits include a reduction in both ventilation days and re-intubations 5. As it is non-invasive, HHFNC also helps to reduce ventilator-associated pneumonia and other lung injuries 5. HHFNC reduce the discomfort, mucosal damage and drying of secretions seen with non-humidified gases 3.
Flows used vary from 1-6L/min, up to 8L/min max. Some units report starting around 5L/min and adjust accordingly. It is recommended that small calibre cannulae are used (obstructing less than 50% of nares) to allow for leak around the nares and thus reduce risk of pressure build-up in the airways 3. The level of pressure generated in each individual is variable and not fully predictable 3
- Infants with Chronic Lung Disease (ability to wean flow over FiO2).
- As a mode of weaning NCPAP support
- Alternative to NCPAP in mild/moderate respiratory distress, for more mature infants.
- Alternative to NCPAP for post-extubation support for more mature infants (28/40 and above)
- Post-op respiratory support.
- Babies with nasal trauma from NCPAP
- Treatment or prevention of apnoea of prematurity.
Consider HHFNC when CPAP 4-6 cmH20 and oxygen requirements <0.4
Change to low flow pernasal oxygen if HHFNC <2-3L and Fi02 <0.3
- Upper airway abnormalities
- Ventilatory failure
- Severe cardiovascular instability
- Frequent apnoea (despite caffeine in preterms)
- Delivery of optimally humidified and warm gases
- Decreased work of breathing
- Decreased body energy expenditure
- Less problems with thickened secretions
- Less problems with nasal irritation/septal damage
- Similar effectiveness in initial management of mild RDS, prevention of intubation/re-intubation without increasing time spent in hospital
- Patient/parent friendly
- Currently there is no way of monitoring end expiratory pressure and there is a theoretical risk of overdistension, lung injury and pneumothorax.
- Gastric distension
- Previous concerns over infection with Ralstonia and gram negative bacteria (see later)
- Prongs: MUST be smaller than 50% of patient’s nares (tight fit of nasal cannulae may generate pressure of 6-10cmH2O at flow as low as 1.5-2 L/min) see images below

- Flow 4-8 L/min (lower flow 5-6 L/min may be sufficient for smaller babies, flow rates > 6 L/min in infants < 1 Kg should be discussed with the duty consultant)
- FiO2 <0.4
- If FiO2 >0.4, increased work of breathing or respiratory incidents, increase flow by 1L/min every 30-60minutes judged by clinical response, until maximum of 8L/min flow
- Operating Temperature:
- Vapotherm - 34 - 35º C for flow rate < 5 L/min and 36 - 38ºC at > 5 L/min
- Optiflow – humidifier set at 37oC (NB – use non invasive setting on the Fisher & Paykel MR850 humidifier)
- Fabian – humidifier set at 37oC(NB – use non invasive setting on the Fisher & PaykelMR850 humidifier)
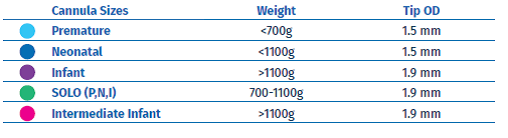
- Cannulae Size - With all the cannulae the weight guides will help select cannula size but the fit should always be double checked to ensure that the cannulae occupy <50% of the nares.
- Vapotherm /Fabian - The same cannulae are compatible with the Vapotherm and Fabian HHFNC devices. Company though recommends to use ONLY Vapotherm cannulae with Precision Flow devices.
The following sizes are a guide but the diameter of nares vary.
| Weight | Cannula Type | Outer diameter |
| < 1.4 Kg | Premature | 0.14 cm |
| 1.4 to 2.6 Kg | Neonatal | 0.19 cm |
| > 2.6 Kg | Infant | 0.27 cam |
From Vapotherm GUIDE (19):
- Optiflow - The following is a guide for Optiflow cannulae with Wigglepad. The cannulae can be used for up to 7 days as per manufacturer. The Wigglepads may be changed independently of the cannulae. The following link contains a demonstration of fitting the cannulae: https://www.fphcare.co.uk/products/optiflow-junior-nasal-cannula
| Nasal Cannulae | Item Code | Approx Weight | Wigglepad code |
| Premature Size | OPT 312 | Up to 3kg | OPT010 |
| Neonatal Size | OPT 314 | 2-8kg | OPT012 |
- The cannulae should rest just below the septum
- If the baby is requiring FiO2 > 0.6 or has CO2 retention, acidosis or frequent apnoea s/he is likely to need alternative support.
- Continuous monitoring of heart rate, respiratory rate and SaO2. Blood gases as indicated if on supplemental oxygen, increasing flow/O2 requirements or on clinical grounds.
-
Consider pneumothorax if baby develops increased respiratory distress and oxygen requirement
Weaning strategies may vary from individual to individual and the following recommendations are a guide only. Weaning strategies fall into two main groups: firstly, those babies with acute respiratory illnesses who may be expected to wean over a shorter timescales and; secondly, infants with chronic lung disease in whom the requirement for respiratory support and supplementary oxygen may be more prolonged.
More mature babies recovering from acute respiratory illness
- Commence weaning once the work of breathing has settled and the Fi02 is falling
- Reduce by 1 L/min every 12-24h if FiO2 < 0.3
- Reduce by 1 L/min every 12h if FiO2 = 0.21
NB – At the discretion of a senior clinician, HHFNC therapy may be discontinued more rapidly than described above if the symptoms of respiratory distress have resolved
Preterm infants with developing chronic lung disease
Babies developing Chronic Lung Disease and higher FiO2 requirements may be prone to atelectasis or apnoeas if the flow is reduced prematurely. They may benefit from a more prolonged period of high flow until their oxygen requirements start to decline with better growth and maturity. Once weaning commences they may benefit from slower rates of weaning than those described above e.g 48hly
- Attempt to reduce by 0.5-1 L/min 24-48 hourly if FiO2 < 0.3
- Attempt to reduce by 0.5L/min 24-48 hourly if FiO2 > 0.3
- Attempt to stop HFNC if requiring 2.0 L/min or less, use LFNC 02 if still in oxygen
After any reduction in the high flow therapy, which results in an increase in the work of breathing or increase in Fi02 by >0.1, consider going back to previous settings
- Non-humidified low flow oxygen therapy may be required after cessation of HHFNC if there is still an oxygen requirement. Remember this is cold and non-humidified gas and may be uncomfortable at higher flow rates
- If an Oxygen flow rate of 1L or greater is required consideration should be given to recommencing HHFNC
Estimated Inspired Oxygen Concentration (%) when using nasal cannulae
|
Flow |
Weight |
|||||||
|
0.7 |
1.0 |
1.25 |
1.5 |
2 |
2.5 |
3 |
3.5 |
|
|
0.01 |
22 |
22 |
22 |
22 |
22 |
21 |
21 |
21 |
|
0.03 |
24 |
23 |
23 |
23 |
23 |
22 |
22 |
22 |
|
0.06 |
28 |
26 |
25 |
24 |
23 |
23 |
23 |
23 |
|
0.125 |
35 |
30 |
29 |
27 |
26 |
24 |
24 |
24 |
|
0.15 |
38 |
33 |
30 |
29 |
27 |
26 |
25 |
24 |
|
0.25 |
49 |
41 |
37 |
34 |
31 |
29 |
27 |
26 |
|
0.5 |
77 |
60 |
53 |
47 |
41 |
37 |
34 |
31 |
|
0.75 |
100 |
80 |
68 |
60 |
51 |
45 |
41 |
36 |
|
1.0 |
100 |
100 |
84 |
74 |
60 |
53 |
47 |
41 |
|
1.25 |
100 |
100 |
100 |
87 |
71 |
60 |
54 |
47 |
|
1.5 |
100 |
100 |
100 |
100 |
77 |
68 |
60 |
51 |
It is possible to deliver nebulized medications in patients receiving HHFNC via Vapotherm Precision Flow. An interface consists of 3 essential parts: Aeroneb ® Pro-X controller, Aeroneb Solo nebulizer (single patient use) and Vapotherm Aerosol Adapter (single use).
Data on efficiency is very limited. It is claimed that in vitro testing demonstrated the inhaled efficiency ranging form 4.5-9.1% at flows 1-8L/min. Another study showed the inspired dose (percent of nominal dose) of 0.6%, 0.6%, and 0.5% for the infant cannula at 3, 5, and 8 L/min, respectively. (Influences of cannula size and flow rate on aerosol drug delivery through the Vapotherm humidified high-flow nasal cannula system Pediatr Crit Care Med. 2013 Jun;14(5):e250-6.)
- For parts and connections required please refer to: APPENDIX 1
The use of inhaled nitric oxide (iNO) in combination with HHFNC is becoming topical and of interest. In newborn infants presenting with hypoxic respiratory failure with hypoxemia without hypercarbia, respiratory distress, or evidence of parenchymal lung disease, iNO in combination with HHFNC may reduce exposure to high levels of inspired oxygen and avoid mechanical ventilation. Randomized and double blinded trials evaluating this therapeutic modality are warranted.
The evidence for inhaled nitric oxide via nasal cannula is limited but neonatal case reports 13 exist, detailing the successful use of inhaled nitric oxide with HHFNC in infants with severe hypoxia and pulmonary hypertension, avoiding the need for mechanical ventilation. It has been postulated that term neonates with idiopathic persistent pulmonary hypertension (PPHN) and adequate respiratory drive without any parenchymal lung disease may benefit from non-invasive methods of iNO delivery to treat the condition without the complications associated with mechanical ventilation. Infants with PPHN suffer from long-term morbidity such as chronic lung disease, neurological morbidity, and sensorineural deafness. Some of these long-term consequences may be secondary to mechanical ventilation. Barotrauma and volutrauma are often associated with prolonged mechanical ventilation in newborn infants. The case of a young infant with primary pulmonary hypertension, who was treated with nasal nitric oxide for several weeks until approved for enrollment into a long-term intravenous prostacyclin therapy program has been reported 13. iNO via a nasopharyngeal tube was used in a 145-day-old infant with a severely hypoplastic lung and end-stage pulmonary hypertension, in whom clinical improvement was maintained for 7 days 13. Premature infants received iNO therapy through continuous positive airway pressure and nasal cannula in the weaning phase in the Nitric Oxide to Prevent Chronic Lung Disease (NO-CLD) trial for prevention of bronchopulmonary dysplasia without any negative consequences 13. Non- invasive delivery of iNO therapy has also been reported for late pulmonary hypertension in newborn infants with congenital diaphragmatic hernia 13. In a case series, patients who had suprasystemic pulmonary hypertension when iNO was discontinued before extubation were treated with iNO through a nasal cannula 13. A large paediatric cardiac intensive care centre in America reports having used inhaled nitric oxide with HHFNC for two years successfully on their patients with pulmonary hypertension and cardiac dysfunction, either avoiding ventilation or facilitating weaning from ventilation and aiding post-op recovery. They have found it to be safe and efficient 12. Furthermore case reports also exist in adult practice detailing the successful use of inhaled nitric oxide and HHFNC is patients with severe pulmonary hypertension and cardiac dysfunction that has improved
oxygenation and haemodynamics thus either avoiding ventilation or allowing weaning from mechanical ventilation 14,15.
Vapotherm offers a Precision Flow Disposable Patient Circuit (DPC) designed for non-invasive nitric oxide delivery via high flow nasal cannula 16. In collaboration with Ikaria®, Vapotherm developed a DPC that is compatible with the INOmax DS & DSIR systems (PF-NODPC-LOW, Nitric Oxide Disposable patient Circuit Low).
It should be noted that increasing the NO setting will increase the NO flow rate, which may cause a bolus of NO2 to be delivered to the patient. This is due to residual NO2 build-up within the INOvent system at very low flow rates. If the NO setting is increased by more than 5ppm in a 5 minute period, a quick purge of the INOvent system should be performed. To perform a quick purge, disconnect the cannula from the sample tee and increase the oxygen flow to 15L/min at the desired NO setting for 1 minute then reconnect at the desired O2 flow setting 17.
- For parts and connections required please refer to: APPENDIX 2
HHFNC systems appears to have similar effectiveness to nCPAP in improving clinical parameters in infants, but at flow rates above 2 L/min. HHFNC are thought to be more comfortable for preterm infants and to cause less nasal septal trauma than NCPAP. From the studies that have reported adverse outcomes, none found a significant increase or difference between HHFNC use vs CPAP use in risk of NEC, pneumothorax, BPD, IVH, ROP, days in hospital or on O2, sepsis or death 2. The use of HHFNC as a primary therapy from birth requires further research.
It is recommend only heated, humidified HFNC systems with flow rates >2 litres/min, up to the maximum flow rate recommended by the manufacturer are used 2. Prongs should not completely occlude the nares. Manufacturers recommend that the prong outer diameter occupy <50% of the nares internal diameter 9. When setting the flow rate, the infant’s weight must be considered as pressure generation increases with decreasing infant size 2. A starting flow rate of 4–6 L/min in newly born preterm infants appears to be a reasonable balance between efficacy and safety based upon the available evidence and current clinical practices.
The majority of units report weaning HHFNC by a gradual reduction in flow rate. Yoder et al 9 recommended increasing the flow rate in 1 L/min increments if (1) FiO2 increased by 0.1 above the starting FiO2, (2) pCO2 increased by 10 mm Hg (equivalent to 1.3kPa) above the baseline value, (3) increased distress or recession noted, or (4) decreased lung expansion noted on CXR. They recommended decreasing the flow rate by 0.5- to 1.0 L/min increments if all of the following were sustained for at least a 4-hour period: (1) FIO2 <0.3 and oxygen saturation within ordered parameters, (2) pCO2 was maintained within ordered parameters, (3) no signs of significant distress were noted, and (4) lung expansion was deemed adequate, if CXR was obtained.
Postulated mechanisms of action
Proposed mechanisms of action include elimination of dead space, reduction in work of breathing, improvement in lung compliance and delivering some degree of CPAP 2, 3.
- Washout of nasopharyngeal cavity
This is thought to be the primary mechanism, reducing the overall dead space and contributing to more effective CO2 elimination. Studies of comparable tracheal gas insufflation demonstrated reduced tidal volume and immediate effect on respiratory rate.
HHFNC reduction of the dead space also has an effect on oxygenation – due to reduced entrainment of room air, the airway oxygen concentration is higher when compared to use of non-rebreathing masks and in patients with mouth opened.
- Reduction of inspiratory resistance
HHFNC matches/exceeds the patient’s inspiratory flow and eliminates increasing nasopharyngeal resistance caused by its inspiratory distendability.
- Warm, humidified gas improves respiratory mechanics
Breathing cold, non-humidified gas for only five minutes decreases lung compliance and conductance. Cold, dry gas elicits bronchoconstriction (nasal mucosa receptors, muscarinic effect).
- Reduced metabolic cost of gas conditioning
The nasopharyngeal cavity provides effective warming and humidification to inspiratory gas but this requires a significant amount of energy. Babies on HHFNC have been shown to gain weight quicker than on CPAP.
- Provision of end distending pressure
High gas flow generates positive airway pressure (although very unreliably and depending on factors like body weight, mouth leaks, etc). It appears that larger babies require higher gas flow in order to maintain effective dead space washout and support inspiratory effort.
Potential concerns
The majority of units use HHFNC in babies of all weights and gestational age. Whilst this modality is popular, the limited evidence available does cause concern amongst clinicians about the exact pressures being delivered to these infant’s lungs and potential for overdistension and injury, particularly infants <1000g 3,4. Pharyngeal pressure delivered by HHFNC increases proportionally, in a linear fashion, when flow is increased 2,4. Pressure delivered to the baby is affected by infant’s weight (higher pressure with lower weights), size/calibre of the cannulae, proportion of nares occluded and if the mouth is open or not 1-4,6. According to more recent studies, pressures generated by HHFNC were similar to or less than those commonly set with nCPAP3. Flow rates of 8L/min have been shown to provide a distending pressure of 4-5 cmH20 7.The Vapotherm unit used in infants has a built -in pressure relief valve, will only deliver a maximal flow of 8L/min and will alarm if high pressure is detected in the circuit 4.
There was in the past a concern regarding potential for infections with Ralstonia spp and gram negative bacteria 1,2,3 which caused a recall of Vapotherm units in 2005. The Vapotherm infection control measures have since become more stringent. Vapotherm’s vapour transfer cartridges now have pores of 0.01 micrometers which are considerably smaller than bacteria (0.2-5micrometers) and thus form a barrier for these pathogens 5.
There has been a case report of a preterm infant who was receiving HHFNC at 2 L/min (device not reported) and concomitantly was found to have subcutaneous scalp emphysema and pneumo-orbitus, which resolved after discontinuation of the HHFNC 2,3. The authors noted that these complications may have been a result of nasopharyngeal trauma rather than the mode of support.
Setup Using Disposable Patient Circuit (DPC)
Refer to Precision Flow Instruction Manual- Setup should be performed by trained personnel only:
- Attach oxygen and air supply to wall outlets
- Open Vapotherm disposable circuit. Connect cartridge to water path unit (either way up), lining up ports. Press firmly into place.
- Attach delivery tube to bottom of water path unit first ensuring that all o-rings are in place
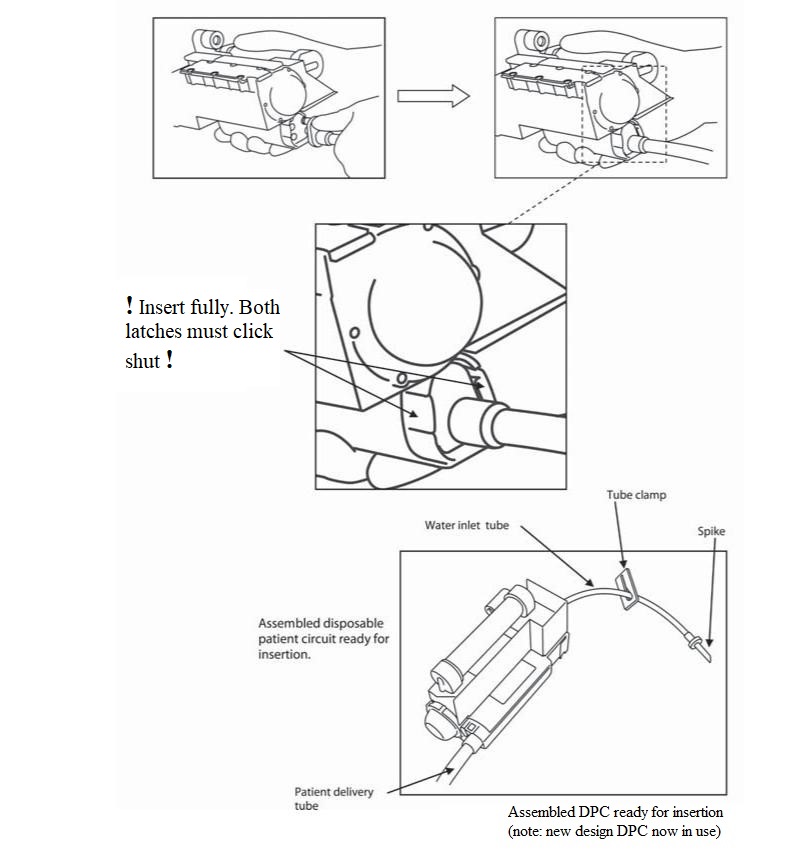
Insert disposable circuit into Vapotherm by sliding door forward, holding unit by handle slide in downwards motion until the unit is firmly in place.
- Close door completely
- Plug in power cord – indicators will light and a self test will be performed. Standby mode starts and Water Out indicator flashes.
- Connect spike to sterile water bag using aseptic technique and release clamp allowing water to flow into the patient water path unit. NB – use easyflow container (sterile water for irrigation). Do NOT use rigid water containers as these would require a vented cap.
- Press Standby / Run to start gas flow, pump and heater. May need to press twice if no display is seen. The unit will bleep during this test. If all tests are passed the unit will enter Run mode and water will flow into patient delivery tube.
- The green flashing LED will change to continuous when desired temperature is reached (from last settings used).
- Adjust settings by pressing the control knob repeatedly for each parameter – to set value turn knob for desired value and press to enter
-
When set temperature reached, attach cannula to delivery tube, then to baby.
Notes:
- Cannula should be half the diameter of nares.
- Low flow cartridge used for preterm babies, neonates and infants (delivers 1-8 lpm).
- When using flow rates less than 5 lpm it is recommended to set temperature no higher than 34° to minimise condensation.
When discontinuing Vapotherm therapy the unit may be left on standby in case HHFNC needs to be resumed. If left standing by the cot side the Vapotherm should be set at these settings :
Flow: 1 L/min,
Temp 33°C,
FiO2 21%
This will keep circuit operational up to 30 days from first use.
Shutting down
- Stop the unit by pressing Standby / Run
- Disconnect from patient
- Clamp the water inlet tube
- Open the sliding door, remove disposable patient circuit, discard as per hospital guidelines
- Disconnect from AC power
- Disconnect gas hoses
Changing disposable circuit
- As above until step 4
- Replace with new patient circuit
- Follow set up instructions
Changing sterile water
- Stop the unit by pressing Standby / Run
- Clamp the water inlet tube
- Attach new sterile water bag, unclamp tubing
- Press Run
Cleaning
- Clean inner housing with Azowipe before and after use
- Clean outer housing and stand with general purpose wipes

- Battery Low or charging
- Disposable water path faulty
- Vapour transfer cartridge type
- Vapour transfer cartridge fault
- Gas supply fault
- Status LED
- Run/ Standby button*
- Setting control knob
- Alarm mute button
- Alarm muted LED
- General fault
- Water out
- Blocked tube
*The vapotherm does not have an on/off switch, when plugged in to wall the battery is charged (15 minutes battery life) and the unit is on Standby/Run.
The disposable patient circuit can be used up to 30 days on single patient.
Delivering nebulised medications via the Vapotherm
Nebulised medications may be given via the Vapotherm circuit using an Aerosol adaptor (Aerogen Aeroneb ®) - Figure 1.
In-vitro studies have demonstrated an inhaled dose efficiency of 4.5 – 9.1%
|
|
Parts required Aeroneb control module (Fig 1 – pole mounted) These are assembled as shown in Fig 2 & 3, The T- piece being inserted between the heated circuit and the nasal prongs. The medication is placed in the nebuliser chamber which must be kept upright, as shown, for the duration of the treatment (adapter at 45°). NB. The T-Piece should be removed between uses as water may condense in the t-piece causing water retention |
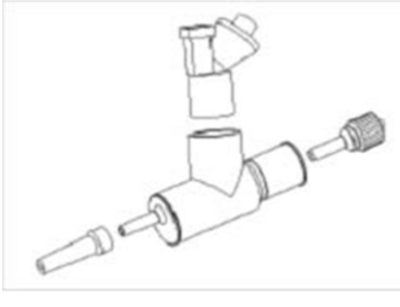 Figure 2 Figure 2 |
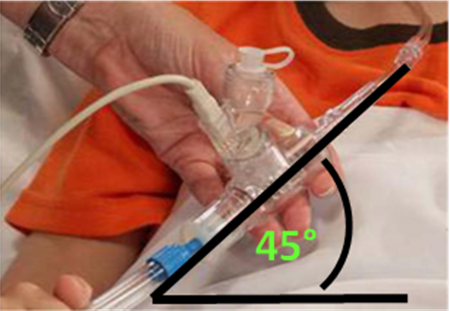 Figure 3 |
Nitric Oxide may be delivered via vie Vapotherm Precision Flow. This requires the following parts
- Precision Flow Nitric Oxide Disposable patient circuit for neonates (low flow)
Part no - PF – NODPC – LOW.
This is inserted into the Vapotherm as per a standard circuit but it has a loop of breathing circuit into which the INOMax Injector module is inserted as shown below
NB – Ensure that this is inserted so that the gas flow follows the arrow on the injector module - A T- piece adaptor to allow for iNO sampling which is inseted between the heated circuit and the nasal prongs as shown below
- iNO concentration is set via the INOMax – see separate guideline for inhaled Nitric Oxide therapy
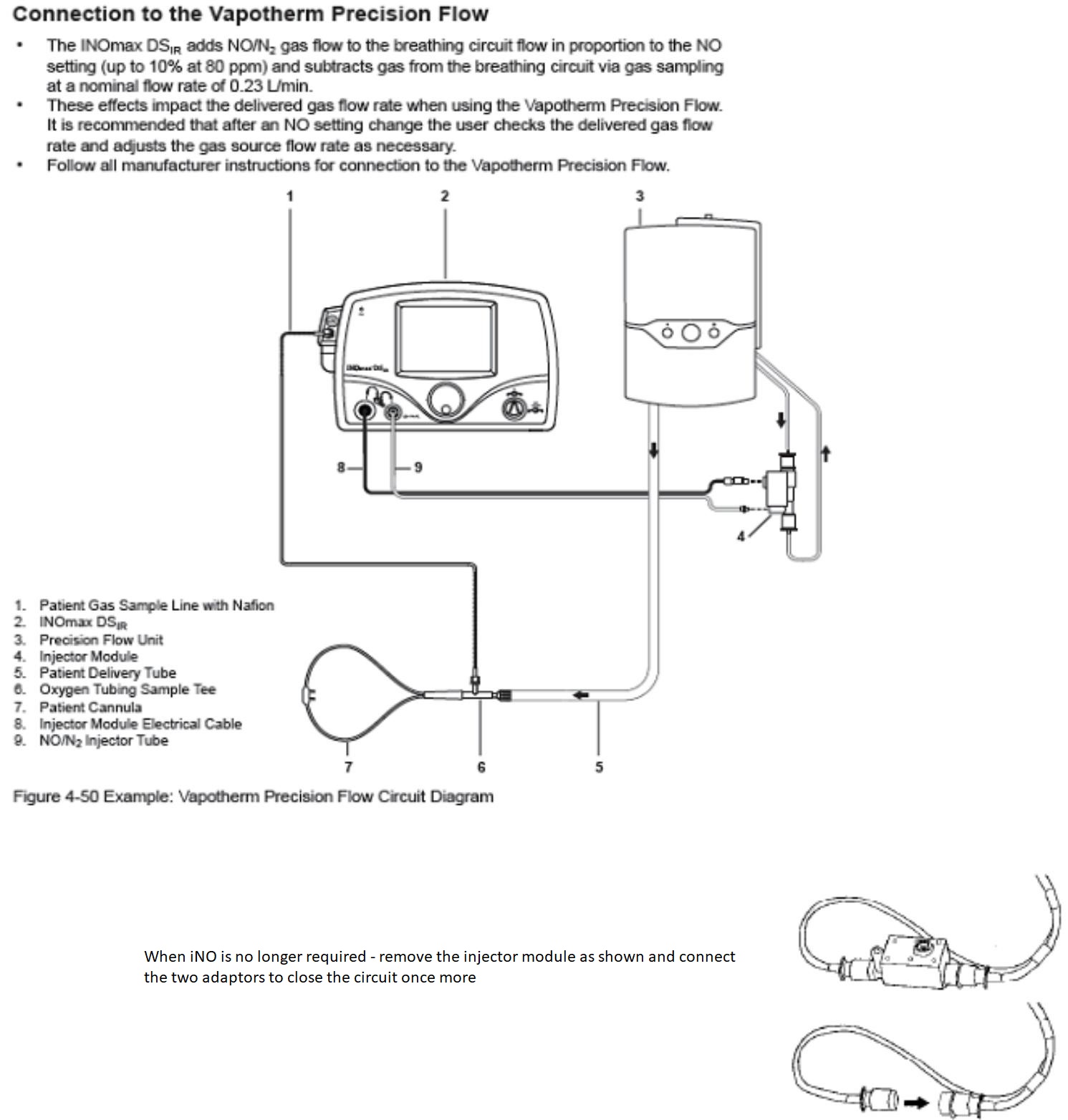
Refer to Optiflow Instruction Manual- Setup should be performed by trained personnel only:
Equipment needed:
- MR850 Heated Humidifer – set to 37 degrees, non-invasive mode MR290 Auto-fill Humidification Chamber Bag of sterile water
- Optiflow breathing circuit
- Air-oxygen blender
- Optiflow Junior Cannulae and wigglepads (size as per instructions on page 3)
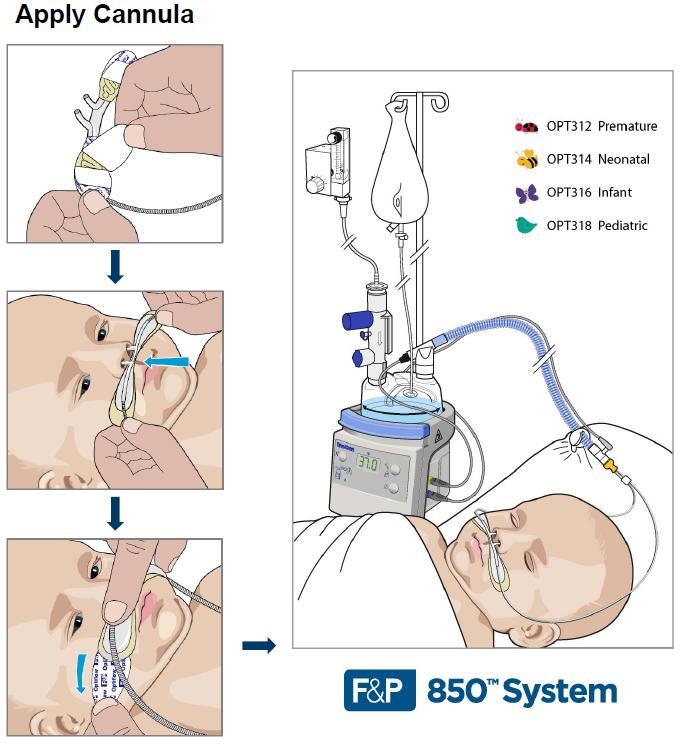
Settings
Humidification – Select Non- invasive mode on FP850 Humidifier
FiO2 – Use blender to select Oxygen concentration
Flow – Use flowmeter to select required flow between 1-8 L/min
Equipment needed:
Fabian HFO 4in1 ventilator OR Fabian therapy Evolution CPAP
Non-invasive breathing circuit – Fig 1 & 2 Humidifier
Fig 1 & 2 – Circuit for High flow – Note in fig 2 an adaptor is required between the breathing circuit and the cannulae due to the different internal diameter
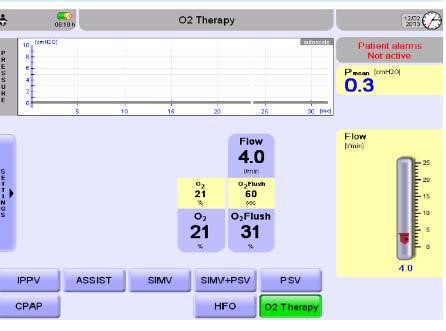
Fig 3 – High Flow Screen on Fabian ventilator
Settings
Humidification – Select Non-invasive mode on FP850 Humidifier
Ventilator setting – ‘O2 Therapy’
FiO2 – Select Oxygen concentration via interface shown and confirm by pressing the control knob
Flow – Select required flow between 1-8 L/min
Pressures generated
- A Test Lung model study (Hasan et al 6) reported that proximal airway pressures using HHFNC were reduced when mouth or nare leaks were present. They tested both the Vapotherm and Fisher Paykel (Optiflow manufacturers) systems. Increasing HHFNC flow resulted in increased cannula and proximal airway pressures. The proximal airway pressures were higher with the Fisher Paykel systems at lower flows but higher with the Vapotherm system at higher flows. The Fisher Paykel system was more affected by moderate leak than the Vapotherm system, resulting in lower proximal airway pressures.
- Collins et al 4 studied the pharyngeal pressures in preterm babies delivered by the Vapotherm and Fisher Paykel HHFNC units. They found no difference in pressure between the two devices at 2-6L/min of flow. At 7L/min flow, Vapotherm delivered a mean (standard deviation) pressure of 4.7 (2.2) cmH20 compared with 4.23 (2.2) cmH20 with Fisher Paykel. At 8L/min flow the mean pharyngeal pressure delivered by Vapotherm was 4.9 (2.2) cmH20 compared with 4.1 (2.3) cmH20 by Fisher Paykel. Maximum pressure recorded for the Vapotherm was 8.6 cmH20, observed at a flow of 7L/min.
Clinical effectiveness
- Vapotherm Precision Flow is one of the commonest units used 2. The device can deliver gases at flow rates 0-40 L/min (2-8 L/min for neonates, using paediatric size vapour transfer cartridge). It generates 3x more water than bubble systems and this is in molecular form (water vapour) delivered at Body Temperature Pressure Saturated (rather than ambient temperature pressure saturated)5
- The 2011 Cochrane review 1, of only 4 small eligible trials, where meta-analysis was not possible, concluded that there was insufficient evidence to establish the safety and efficacy of HFNC in preterm infants at that time. The flow rates evaluated were generally lower (<2L/min) than is used in current practice today. Nair et al 2005 found similar treatment failure rates between CPAP and HFNC when used as primary respiratory support post birth. Campbell et al 2006 reported significantly higher rates of reintubation when on HFNC compared to CPAP following recent extubation, however this was unheated and flows used were <2L/min. Woodhead et al 2006 and Miller et al 2010 reported no significant difference in treatment failure or reintubation between humidified and unhumidified HFNC. Miller et al compared Vapotherm and Fisher-Paykel with no significant difference between each unit. The unhumified group did have significantly more nasal mucosal damage.
- Collins et al 7 randomised 132 preterm infants (<32 weeks) to receive CPAP or HHFNC (Vapotherm) post extubation. They found similar rates between both treatment modalities in terms of extubation failure and need for reintubation, thus concluding one modality did not appear more superior in terms of preventing reintubation in the first 7 days after extubation, even at lower gestations on subgroup analysis. The CPAP group had significantly higher nasal trauma.
- Manley et al 8 studied HHFNC vs CPAP for prevention of extubation failure in over 300 preterm infants in a randomised, multicentred control trial. Their results concluded that HHFNC was statistically no less inferior to CPAP at reducing extubation failure in the first 7 days post extubation. HHFNC flows were 5-6L/min and CPAP pressure 7m H20. It should be noted that in the infants who failed on HHFNC, 50% were successfully treated with CPAP or NIPPV, preventing reintubation. They also found statistically significant higher nasal trauma in the CPAP group. The unit used was Optiflow (Fisher Paykel). There was no increased incidence of adverse effects or complications in either group. Their trial wasn’t powered for subgroup analysis of extremely preterm infants but they did find a trend favouring CPAP to reduce extubation failure.
- Yoder et al 9 undertook a multicentre RCT of over 400 infants from 28-42 weeks gestation, comparing HHFNC (Fisher Paykel and Vapotherm) with CPAP as a treatment modality post birth as primary therapy or post extubation. The primary outcome was defined as a need for intubation within 72 hours of applied noninvasive therapy. There was no statistically significant difference in early failure for HHFNC versus nCPAP, subsequent need for any intubation, or in any of several adverse outcomes analysed, including air leak. HHFNC infants remained on the study mode significantly longer than nCPAP infants (median: 4 vs 2 days, respectively), but there were no differences between study groups for days on supplemental oxygen, bronchopulmonary dysplasia, or discharge from the hospital on oxygen. The concluded that among infants >28 weeks’ gestational age, HHFNC appears to have similar efficacy and safety to nCPAP when applied immediately postextubation or early as initial non-invasive support for respiratory dysfunction.
- Holleman-Duray et al 2 compared HHFNC to NCPAP and found no difference in rates of extubation failure. They also reported less ventilator associated pneumonia and better growth in the Vapotherm group.
- Woodhead et al 2006 5 reported no re-intubations with Vapotherm. However when a standard high-flow nasal cannula was used, two infants had to be re-intubated and five were switched to Vapotherm. Improved blood gases on Vapotherm, along with a reduction in the fraction of inspired oxygen requirement, apnoeas and bradycardias were also reported. They reflected that due to the temperature and high relative humidity, the flows delivered by Vapotherm do not dry the baby's nasal passages and also reported that respiratory effort was lower on Vapotherm and, therefore, more effective than standard high-flow nasal cannula.
- Shoemaker et al 2 found that more infants were intubated for failing NCPAP compared to HFNC (40 vs. 18%) following the introduction of HFNC. Ventilator days per patient were decreased (mean 19.4 vs. 9.9 days) following the introduction of HFNC as early respiratory support.
- Saslow et al 2 conducted a crossover study of preterm neonates who were medically stable on HFNC or NCPAP for treatment of mild RDS, bronchopulmonary dysplasia (BPD) or AOP and showed no difference in tidal volumes on either device.
- The study of Lampland 2 et al compared NCPAP at 6 cm H2O to HFNC at 1–6 litres/min in a group of 15 preterm infants with stable RDS and showed only the development of tachypnoea at HFNC <2 litres/min.
- Sreenan et al 2 also compared HFNC to NCPAP for AOP and showed no difference in apnoea severity.
- Abdel-Hady et al 2 randomised 60 preterm infants (>/= 28 weeks’ gestation) who were stable on nCPAP at 5 cm H20 with fraction of inspired oxygen <0.30 for at least 24 h into two groups. The nCPAP group was kept on nCPAP until they required no supplemental oxygen for 24 h and then placed in air. The HFNC group was changed to humidified HFNC at 2 litres/min. This flow rate was maintained until no supplemental oxygen was required, then decreased by 0.5 litres/min every 6 h to 0.5 litres/min. The HFNC group had a clinically important increase in days on oxygen (median 14 vs. 5 days) and duration of respiratory support (18 vs. 10.5 days; p = 0.03).
- Iranpour et al 2 randomised preterm infants (30–35 weeks’ gestation) 24 h after early surfactant treatment and immediate extubation to NCPAP to either continuing NCPAP at 6–8 cm H 2 O or changing to Fisher & Paykel HFNC. There were no differences between the groups with regard to short-term morbidities, duration of hospitalisation or duration of oxygen treatment.
- Roark et al 2006 5 found that only 27 per cent of patients required some sedation on the Vapotherm, concluding the device was well-tolerated.
- Wilkinson D, Andersen C, O’Donnell CPF, De Paoli AG, High flow nasal cannula for respiratory support in preterm infants (review), The Cochrane Collaboration, The Cochrane Library, 2016, issue 5.
- Ojha S, Gridley E, Dorling J, Use of heated humidified high-flow nasal cannula oxygen in neonates: A UK wide survey, ActaPaediatrica, 2012, 102, pp 249-253
- Manley B, Dold S, Davis PG, Roehr C, High-flow nasal cannulae for respiratory support of preterm infants: A review of the evidence, Neonatology, 2012, 102:300-308.
- Collins C, Holberton JR, Konig K, Comparison of the pharyngeal pressure provided by two heated, humidified high-flow nasal cannulae devices in premature infants, Journal of Paediatrics and Child Health, 49 (2013), 554-556
- Vapotherm Operating Instructions [Staffnet link]
- Hasan R, Habib R, Effects of flow rate and air leak at the nares and mouth opening on a positive distending pressure delivery using commercially available high-flow nasal cannula systems: A lung model study, PediatrCrit Care Med 2011, Vol 12 No 1
- Collins C, Holberton JR, Barfield C, Davis PG, A randomised control trial to compare heated humidified high flow nasal cannulae with nasal continuous positive airway pressure post extubation in premature infants, J Pediatr 2013, 162, 949-54.
- Manley BJ, Owen L, Doyle LW, Andersen C, Cartwright D, Pritchard MA, Donath S, Davis PG, High-flow nasal cannula in Very Preterm Infants after Extubation, New England Journal of Medicine, 2013, 369;15,pp 1425-1433
- Yoder BA, Stodder RA, Li Ma, King J, Dirnberger DR, Abbasi S, Heated, Humidified High-Flow Nasal Cannula Versus Nasal CPAP for Respiratory Support in Neonates, Pediatrics 2013;131:e1482–e149
- http://www.rcecs.com/MyCE/PDFDocs/course/V7122.pdf [Link no longer live: an archived version can be found here: https://web.archive.org/web/20151006040511/http://www.rcecs.com/MyCE/PDFDocs/course/V7122.pdf]
- UTMB Policies & Procedures. Operating Instructions for Inhaled Nitric Oxide Therapy.
- UTMB Policies & Procedures. Institutional Handbook of Policies and Procedures.
- Boyle KM, Nitric Oxide Delivery Via Nasal Cannula. RT, Feb 2007.
- Nair J, Orie J, Lakshminrusimha S, Successful Treatment of a Neonate with Idiopathic Persistent Pulmonary Hypertension with Inhaled Nitric Oxide via Nasal Cannula without Mechanical Ventilation, AJP Rep. Nov 2012; 2(1): 29–32.
- Huang J, Fridman D, High Flow Oxygen and Low Dose Inhaled Nitric Oxide in a Case of Severe Pulmonary Hypertension and Obstructive Shock, Chest. 2011;140 (4_MeetingAbstracts):63A. doi:10.1378/chest.1117113
- Gauran C, Retherford L, Goswami S, High flow nasal cannula oxygen and nasal inhaled nitric oxide used for mechanical ventilation weaning in severe right ventricular failure
- http://cdn.vtherm.com/public/246/documents/Version-20131001070755-Documents-246-9886-1.pdf [Link no longer live]
- http://ikariaaust.com/sites/default/files/Update10_nasal_cannula.pdf ACCESSED 14/05/2014
- Fisher & Paykel Healthcare. Nasal High Flow Therapy.
- http://www.inspiration-healthcare.com/downloads/quick-setup-guide-57.pdf [Link no longer live]
- Vapotherm. Neonatal Guidelines and Best Practices for High Flow Nasal Cannula (HFNC)
Last reviewed: 25 October 2024
Next review: 31 October 2025
Author(s): Dr Tomasz Dygas – Consultant Neonatologist PRM; Dr Jennifer Mitchell- Consultant Neonatologist QEUH
Approved By: West of Scotland Managed Clinical Network for Neonatology